Getting Started
Run in Postman™
Below is a collection with all examples from this API release. You can use this collection locally in the Postman™ application or in a Postman™ for Web workspace.
For local Postman, use the Get App link in the collection import dialog, or visit the Postman™ website Once you click the Run in Postman button to download the collection, you can choose which workspace to import it into.
If successfully imported, the collection displays (example below) with folders, each containing example API. The folders follow the naming / navigation of this reference page.
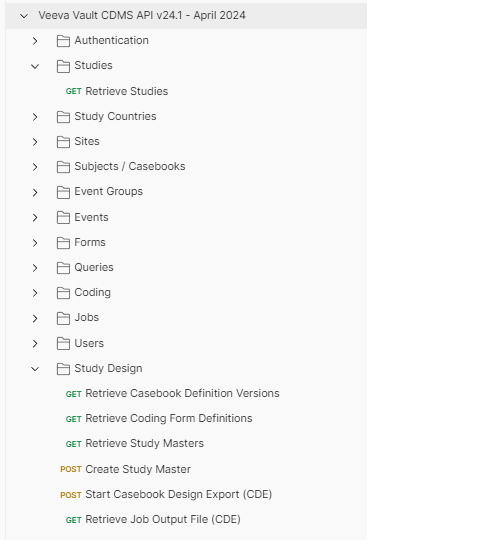
To use this collection effectively, use environments to setup a series of variables. The following environment variables are embedded in the collection endpoints:
-
vaultDNS: DNS of the Vault. Do not include a trailing slash (/), or the prefix of the URL (https://) -
username: your Vault username -
password: your password to that Vault account -
version: API version
The screenshot below shows environment variables for the following Vault setup:
- Vault DNS: https://myvault.veevavault.com
- User Name: jsmith@verteopharma.com
- Vault Version: 24.1
EDC Overview
Data Organization
Data for a EDC Study follows a model similar to CDISC ODM, e.g. Visits / Forms / Item Groups / Items. EDC, however, terms a Visit instead an Event, and Event Groups can group a series of Events.
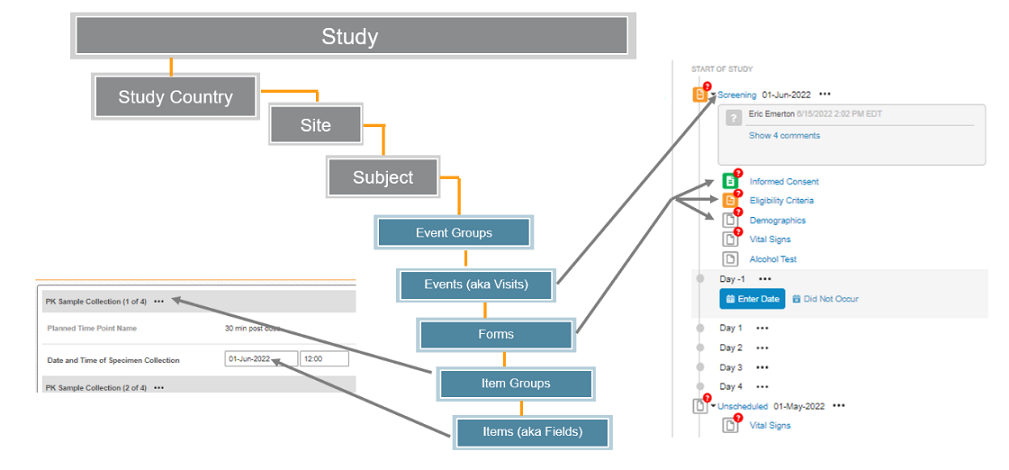
| Data Level | Notes |
|---|---|
Study |
Main component of EDC. A single Vault typically contains many Studies |
Study Country |
Collection of Sites in the Study |
Site |
Collection of Subjects in the Study at that location |
Subject |
A patient in the Study, their data collected across all visits in the Study |
Event Group |
Collection of Events, possibly repeating. Examples Screening Period, Treatment Cycle, Unscheduled Visit |
Event |
One to one with a visit for a Subject, the data collected at that visit. Examples - Screening, Visit 1, Visit 2 |
Form |
Organized data for the Subject at an Event, possibly repeating. Examples - Vitals, Demography, PK, etc. |
Item Group |
Organized set of Items on a Form, possibly repeating. |
Item |
A single data point for the Subject. Examples - Date of Birth, Weight, Adverse Event Description |
Study Design and Operations
- Study design work originates in a Development Vault, with subsequent deployments to Test/UAT Vault, and eventually a Production Vault.
- Study design can involve dynamic Rules, who will add appropriate Event Groups, Events and/or Forms, on the submit of Forms that satisfy specific conditions. The API can also add new Event Groups, Events, Forms, perhaps exclusively, or it can also submit existing Forms to execute the dynamic Rules.
- Access within a Study follows the Study Country -> Site hierarchy, i.e. which Sites a user can see. A Data Manager might have access to all, a regional CRA some countries but not all, and site users just their specific Site.
- Common Forms (also known as Log Forms) are a special type of repeating Form that aren't associated within a specific Event. Examples include Adverse Events and Concomitant Medications.
- Given this example Study:
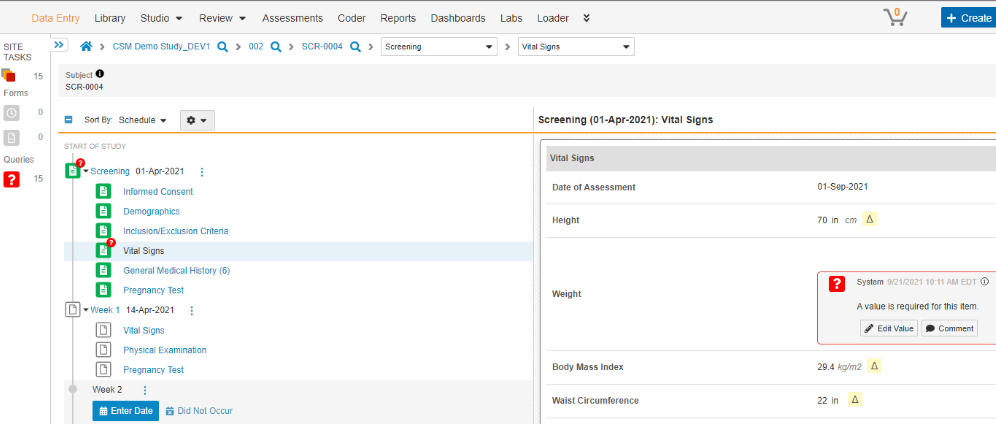
- Study = CSM Demo Study_DEV1
- Study Country = (not pictured, United States, a relationship to the site)
- Site = 002
- Subject = SCR-0004
- Event Group = (not pictured). A group of Events (aka visits) are organized into an Event Group to better facilitate repeating cycles and unscheduled Events.
- Event = Screening
- Form = Vital Signs (details, right side of pictured)
- Item Group = Vital Signs (group of fields on a CRF, used for repeating when necessary)
- Items = Date of Assessment (date field), Weight (unit codelist field), etc.
EDC API Overview
- The EDC API is based on the platform Vault API and designed for English locale users.
- The GA label of this reference refers to the most recent major/general release
- The Beta label sections of this reference refer to the coming major/general release.
- Execution of Beta API(s) will only work on either (i) Pre Release Vaults (available approximately 4 weeks from general releases), or (ii) Limited Release Vaults (releases approximately every month, in between general releases).
- Veeva Vault releases three new API versions each year, coinciding with Vault general releases. The release cycle is YY.1 (April), YY.2 (August), and YY.3 (December) of each year. For example, the first Vault General Release of 2024 is 24R1. The API version which coincides with this release is API v24.1. The third Vault General Release of 2023 is 23R3, which coincides with REST API v23.3.
- The EDC API was first available at the Vault / Clinical Data 19R3 release.
- Other Vault applications (Safety, Clinical Operations, etc.) have application specific APIs, just as Clinical Data does.
- To view the new APIs, features, and fixed issues for each API version, you can check the Developer Release Notes.
- Integrations written on specific versions do NOT have to necessarily move forward with a new release of the API. The downside is that new features and enhanced abilities of existing APIs won't be available in those older releases.
- If you plan to move an integration forward regarding its API release, always perform appropriate testing in pre release and/or limited release Vaults prior to applying to general release / production Vaults.
Structuring the Endpoint
The following variable notation is used in the endpoint examples throughout this page:
-
{vaultDNS}: the domain name of your Vault. You can find this by logging into your Vault through the user interface. For example, upon logging in, if the URL is https://my-vault.veevavault.com/ui/ the{vaultDNS}value is my-vault.veevavault.com. If you have access to multiple Vaults on a single domain, the user account and login endpoint are the same, but the{vaultDNS}part will be different for interacting with each of the specific Vaults (after API login). -
{version}: API version. Replace it with a version number, such as v24.1. For example, https://my-vault.veevavault.com/api/v24.1. The older versions of the API can continue to be used on later versions of the overall Vault Release.
API Access and Roles
- To use the EDC API, you first need a Vault user account. That account must be assigned a Study Role that grants at least the API Access permission.
- The role permissions for the API user (via its role) typically match those required to perform the same action in the EDC UI. For example, to use the Combination Update Form Data endpoint, you must have the View Casebook (view Subject) and Data Entry permissions, in addition to the API Access permission.
- Clinical Data has two template or standard roles for API users: CDMS API Read Write and CDMS API Read Only.
- The CDMS API Read Write template role can perform all actions. For example, adding queries, closing queries, creating subjects, updating form data, managing users, etc.
- Due to some of the endpoints requiring full access in a study, a user using the template role CDMS API Read Write must have All Sites access in a Study. This is not the case for users using the lesser role CDMS API Read Only.
Custom Roles
- To lessen the actions of an API user, a custom role is necessary.
- Start with a new role that is a copy of CDMS API Read Write, and refer to the table below for which permissions to remove, i.e. the actions you do not want that API user to be able to perform.
- For an API user that will have access to some but not all Sites in the study, the custom role must exclude the permissions noted in the table where User Must Have All Sites Access? is YES.
- Your organization may have configured your Vault with custom roles, based on the standard ones, so discuss with your organization's administrator what permissions your API-level accounts require.
For more information on the setup of custom roles, standard roles, and user accounts, see Managing Study Roles and Managing User Access inClinical Data Help.
| Permission in Role Management Grid | Description | User Must Have All Sites Access? |
|---|---|---|
| API Access | Ability to access and use the Vault EDC API | No |
| View Casebook | Ability to view information about Subjects (their Casebooks). Generally, every user should have this permission. | No |
| Add Casebook | Ability to create new Subjects / Casebooks | No |
| Data Entry | Ability to enter study data into Casebooks, including entering Event Dates, setting Item values, and submitting Forms | No |
| Assign Code | Ability to view set suggestions and/or coding on Medical Coding Requests | YES |
| Open Query | Ability to open (create new) queries or reopen a closed Query | No |
| Answer Query | Ability to answer (comment on or respond to) queries | No |
| Close Query | Ability to close queries | No |
| Manage Users | Ability to create and edit user accounts and assign users their Study Roles | YES |
| Manage Email Group Assignment | Ability to manage memberships of users in email groups of a study (part of user management) | YES |
| Edit Study Sites | Ability to create and edit Sites in the Study | YES |
| Design Study | Ability to create studies, setup the study schedule, copy forms to the new study, etc. | YES |
| Design Library | Ability to create library studies, copy forms to the new library study, etc. | YES |
| Manage Safety Configuration | Relates to the Design Study permission | YES |
Insufficient Access
If you don't have API access, your authentication request might succeed, but any other API calls you make will return the following error (one example):
INSUFFICIENT_ACCESS: User [ID] not licensed for permission [VaultActions_API_Access].
Contact your Vault administrator to adjust your study account(s) or role(s) for API access.
Login / Sessions
Request
curl -X POST -H "Content-Type: application/x-www-form-urlencoded" \
-d "username={username}&password={password}" \
"https://my-vault.veevavault.com/api/v24.1/auth"
Response
{
"responseStatus": "SUCCESS",
"sessionId": "802E62F765575BEB70642BE7A822A419F48B41312ECCAF4767D8DD956873DEE90D677F053A5DAB00B37E2C6B42FA6B15FCE6147C6120F56A638D911EBDFA007B",
"userId": 92677,
"vaultIds": [
{
"id": 1004329,
"name": "My Vault",
"url": "https://my-vault.veevavault.com/api"
},
{
"id": 1004330,
"name": "My Vault 2",
"url": "https://my-vault2.veevavault.com/api"
}
],
"vaultId": 1004329
}
Your first API call will be an authentication request, which is the same endpoint for the access of any Vault. Successful login provides back a session ID for other API calls to include. In the response example to the right, this is the value 802E62F76557....BDFA007B.
- For username/password style login, which is the most common, the endpoint
/api/{version}/authexpects a POST attempt with two URL-encoded form parameters (x-www-form-urlencoded):usernameandpassword. GET / URL style parameters to the authentication are specifically disallowed. - The
vaultIdscollection in the response is a list of available Vaults for which the user has access into. - The
vaultIdfield under the list of available Vaults tells you which Vault you’ve authenticated against. Rarely, can you authenticate against the wrong Vault if the intended Vault is inactive. In this situation, you’ll authenticate against the last Vault you accessed or the oldest Vault in your domain. Check thevaultIdagainst the information in the list to ensure that it’s the Vault you intended, as you begin other requests. - If the specified Vault is invalid or inactive, a session ID may be returned for an alternate default Vault. It’s important to verify that the currently authenticated Vault shown by the
vaultIdfield is the expected one.
Session Duration
A session is considered "active" as long as some activity (either through the UI or API) happens within the maximum inactive session duration. This maximum inactive session duration varies by Vault, and is configured by your Vault Admin. For example, a Vault configured with a session duration of 10 minutes means a session will expire 10 minutes after the last API request finishes executing. As long as an API request is active, the session that made that API call will continue to be valid until the request finishes executing. If you are a Vault Admin, learn more about configuring Session Duration in Vault Help.
The default session length is 20 minutes. The maximum session duration is 48 hours, which is not configurable. This means that even if you keep your session active through activity, it cannot remain active for longer than 48 hours. To keep sessions valid in a long-running integration, see our best practices.
In addition, a session could be invalidated based on major security changes. For example, a password change or account deactivation.
Best Practices
We recommend reusing the sessionId obtained after login to execute as many API requests as possible. To keep your session active, use the Session Keep Alive endpoint. This avoids unnecessary auth calls, which helps your integration stay within the API rate limits.
Make sure your integration catches invalid session exceptions and obtains a new session as needed. Your integration should also include a hard expiration at 48 hours, which is the maximum session duration.
Pagination
Request - Retrieve Queries (1750 in the study) with default page size of 1000
$ curl -X GET -H "Authorization: {SESSION_ID} \
https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/?study_name=ABCP-2022-01_DEV1
Response - 1st Page (Results 0-999)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 1000,
"total": 1750,
"next_page": "/api/{version}/app/cdm/queries?resource_locator=4db7ac7f-aa08-486a-99e1-9acb5cdda80e&limit=1000&offset=1000",
"resource_locator": "4db7ac7f-aa08-486a-99e1-9acb5cdda80e"
},
"queries": [
:
:
(1000 entries are here, one per query)
:
:
]
}
Request - Retrieve Queries, 2nd Page
$ curl -X GET -H "Authorization: {SESSION_ID}" \
https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/resource_locator=4db7ac7f-aa08-486a-99e1-9acb5cdda80e&limit=1000&offset=1000"
Response: 2nd Page (Results 1000 - 1749)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 1000,
"size": 750,
"total": 1750,
"previous_page": "/api/{version}/app/cdm/queries?resource_locator=4db7ac7f-aa08-486a-99e1-9acb5cdda80e&limit=0&offset=0",
"resource_locator": "4db7ac7f-aa08-486a-99e1-9acb5cdda80e"
},
"queries": [
:
:
]
}
By default, Vault returns a maximum of 1000 results per page. This means that for a paginating API, if there are more than 1000 results to be retrieved, you must send multiple API requests to retrieve all data.
Paginating APIs return a json node called responseDetails, which contains the current limit, offset, size, and total.
limit= maximum page size (defaults to 1000)offset= offset into result set (first result is offset 0)size= size of current pagetotal= total # of results to be returned across ALL pages
If additional pages are available, the responseDetails node includes a next_page and resource_locator (an identifier of the overall query) fields. Whenever the next_page field is present in the API response, this indicates that additional page(s) are still available and must be separately retrieved. When fetching a next_page, there is no need to provide any of the original API parameters (like study_name, study_country, etc.). The only required parameters when using next_page are the limit, offset, and resource_locator, which are automatically included in the next_page value.
To recap, next_page requests must contain an offset (positive integer or zero), a limit (positive integer less than 1000), and a resource_locator, all of which are included in the next_page URL returned by a paginating API.
For the example on the right (a call to the retrieve queries endpoint, for all queries in the study, 1750 in total):
- The initial API request would result in 1000 total queries being returned
- We can see this because the
limitin the response is set to the default value of 1000 - The
size(indicating the current page size) is also 1000, yet thetotalnumber of results to be returned is 1750 (across all pages) - Additionally, we see that the
next_pageresponse field is present.
Users can also set their own limit and offset by providing limit and offset parameters along with the initial API request. In this case, the next_page and previous_page fields returned will reflect the user desired values
Rate Limits
API rate limits are a common way to guarantee a high-quality service by preventing servers from becoming overloaded and the web service itself from becoming unusable. Web services are a fundamental resource to any application or integration. Vault enforces rate limits to ensure that each application or integration gets its fair share of this resource. Learn more about API rate limits in Vault Help.
How Does Vault Calculate Limits?
Vault enforces multiple types of rate limits:
Burst Limit: The number of API calls that your Vault can receive within a fixed 5-minute period. When you reach the burst limit, the server delays responses for the remainder of the burst-limit period. To determine the length of delay for a throttled response, check the
X-VaultAPI-ResponseDelayresponse header value.Auth Burst Limit: The number of calls that your Vault can make to
/api/{version}/authin a one (1) minute period. When you reach 50% of the burst limit, the server delays responses for the remainder of the burst-limit period. This limit is tracked by theusernameandvaultDNSparameters and does not apply to SAML/SSO or OAuth authentication. To determine the burst limit for your Vault or the length of delay for a throttled response, check the response headers or API Usage Logs (available in the Admin UI of Vault). WARNING: The limit for logins via the API in any one calendar minute is 20.Job Status: The Job Status endpoint will return the
API_LIMIT_EXCEEDEDerror if requested more than once in 10 seconds. When polling for a job's state, be sure to throttle your requests.
For example, a Vault might allow 2,000 API requests within a 5-minute window. Between 4:00 and 4:03, your Vault has received 2,000 requests. On request 2,001 at 4:04, the server slows down all requests until the next window begins at 4:05.
As of v21.1, Vault no longer enforces daily API limits, including the daily_limit_remaining in API usage logs, or sends notifications to users when API transaction limits are partially reached or exceeded.
APU Rate Limit Headers
Vault APIs return rate limiting headers to help you monitor how many API calls you have remaining as well as possible response throttling.
X-VaultAPI-BurstLimit: Indicates the maximum number of calls allowed in a 5-minute burst window. For example, 2000. (Included in v19.2+)X-VaultAPI-BurstLimitRemaining: Indicates the number of API calls remaining for the current 5-minute burst window. For example, 1945, i.e., 55 have been used of 2000 in the current 5-minute interval. (Included in v14.0+)X-VaultAPI-ResponseDelay: Indicates the delay, in milliseconds, of a throttled response. Only included for delayed responses. For example, 500ms. See How Does Vault Calculate Limits?. (Included in v14.0+)
As of v21.1, Vault APIs no longer returns the X-VaultAPI-DailyLimit or X-VaultAPI-DailyLimitRemaining headers. To ensure backwards compatibility, Vault APIs v20.3 and below still return the headers with a value of 999,999. Vault does not deduct from this value with each request.
Authentication Rate Limit Headers
As of v20.1, calls to /api/{version}/auth return two rate limit headers in every response showing you the total limits allowed for your Vault and how many /api/{version}/auth calls you have remaining. These calls also count towards your burst and daily limits.
X-VaultAPI-BurstLimitRemaining: Indicates the number of API calls remaining for the current 1-minute burst window. For example, 19.X-VaultAPI-BurstLimit: Indicates the maximum number of calls allowed in a 1-minute burst window. For example, 20.X-VaultAPI-ResponseDelay: Indicates the length of delay for a throttled response in milliseconds. For example, 2000.
Developing with Rate Limits:
Here are some best practices for reducing the number of API requests:
Avoid unnecessary
authcalls. A session ID with a timeout of 20 minutes only expires if it is not used within 20 minutes after the last request finishes executing.Cache configuration data. Configuration data does not change often. Retrieve it once and store it locally in a database like SQLite or serialized in a file.
Optimize your code to eliminate unnecessary API requests. Are you retrieving data that isn’t being used in your application? Are you updating data that hasn’t changed? Eliminate these calls.
Regulate the API request rate. If you are frequently approaching or reaching the API rate limit, consider implementing a throttling process in your application to distribute your requests more uniformly over time. For example, observe the above-mentioned response headers and monitor your request rate. Throttle requests when your rate reaches a certain threshold.
Client ID
Recommended Client ID Format
{company}-{organization}-{component/team}-{server | client}-{program}
For additional tracking purposes, every Vault REST API call will accept an optional client ID to represent an external integration client. You can provide this data via a query parameter called client_id or HTTP Header calledX-VaultAPI-ClientID. If client ID is included as both a HTTP Header and query parameter, the query parameter is ignored.
The Vault API will record the client ID value for each API call. This ID appears in the API Usage Logs, which is downloadable through the UI and API. If an API request does not include a client ID, the value will appear as unknown in the API Usage Log. The API Usage Log is only available in v18.1+, but client ID can be included in requests for all versions of the API.
Example: Vault Loader Team Client ID
veeva-vault-tools-server-loader
A valid client ID must be an alphanumeric string with a maximum of 100 characters. A client ID can be mixed-case and the only special characters allowed are underscores _ and hyphens -. If an API request includes an invalid client ID, the value will appear as invalid_client_id in the API Usage Log.
To avoid clashing with other integrations or client applications running on Vault, we recommend that you format you use our recommended Client ID format.
Errors - General
Example: Failed Authentication
{
"responseStatus" : "FAILURE",
"errors" : [
{
"type" : "NO_PASSWORD_PROVIDED",
"message" : "No password was provided for the login call."
}
],
"errorType" : "AUTHENTICATION_FAILED"
}
Example: Down for Maintenance
{
"responseStatus": "FAILURE",
"responseMessage": "Authentication failed for user [chunter@abcpharma.com]",
"errors": [
{
"type": "DOWN_FOR_MAINTENANCE",
"message": "Vault is currently down for maintenance"
}
],
"errorType": "AUTHENTICATION_FAILED"
}
The response of every API call includes a field called responseStatus. Possible values are:
-
SUCCESS- The API request was successfully processed. -
FAILURE- The API request could not be processed because of user error. -
EXCEPTION- The API request could not be processed because of system error.
For a responseStatus other than SUCCESS, users can inspect the errors field in the response. If the errors are returned in an errors JSON array (depends on the level of fail, EDC or Vault), each includes the following fields:
type- The specific type of error, e.g.,INVALID_DATA,PARAMETER_REQUIRED, etc. See below for a full list of types. These values are not Subject to change for a given version of the API, even when newer versions of the API are available.message- The message accompanying each error type, e.g., Missing required parameter [{field_name}]. When available, the error message includes the specific reason, e.g., the {field_name} for the error. These messages are Subject to change and are not contractual parts for error handling.
| Type | Description |
|---|---|
UNEXPECTED_ERROR |
General error catch-all when there is no specific error, or an unidentified error. |
MALFORMED_URL |
The specified resource cannot be found. |
METHOD_NOT_SUPPORTED |
The specified resource does not support the (POST, PUT, GET, DELETE) method. Or, the API request is not supported by this version of API. |
INACTIVE_USER |
User is found, but not active. |
NO_PASSWORD_PROVIDED |
No password was provided for the login call. |
USERNAME_OR_PASSWORD_INCORRECT |
Authentication failed because an invalid username or password was provided. |
USER_LOCKED_OUT |
The user is locked out. The only way to unlock a user account is for another administrator to perform the Reset Password operation on the user. |
PASSWORD_CHANGE_REQUIRED |
Password change required. |
INVALID_SESSION_ID |
Invalid session ID provided. |
DOWN_FOR_MAINTENANCE |
The Vault is currently down for maintenance. |
PARAMETER_REQUIRED |
Missing required parameters in the API call. |
INVALID_DATA |
Invalid data provided in the API call. |
INSUFFICIENT_ACCESS |
User does not have sufficient privileges to perform the action. Additionally, the ../actions/.. endpoints may return this error in cases where the user attempts to access a resource which does not exist. |
OPERATION_NOT_ALLOWED |
Certain rules that must be met to perform this operation have not been met. |
ATTRIBUTE_NOT_SUPPORTED |
The specified resource does not recognize provided attributes. |
INVALID_FILTER |
Provided a non-existent filter to Retrieve Documents. |
INCORRECT_QUERY_SYNTAX_ERROR |
Query string used with VQL has an incorrect query syntax. |
RACE_CONDITION |
A rare condition where the same record is being simultaneously updated by another API call. |
EXCEEDS_FILE_MAX_SIZE |
The size of uploaded file exceeds the maximum size allowed (4 GB). |
API_LIMIT_EXCEEDED |
Vault limits the number of API calls that can be made every 5-minutes and every 24 hours. Additionally, the Job Status endpoint can only be requested once every 10 seconds. When either of these limits are reached, this error message is returned, and no further calls will be processed until the next 5-minute or 24-hour period begins. Learn more about API Limits. |
CONFIGURATION_MODE_ENABLED |
Non-Admins cannot access a Vault in Configuration Mode. Learn more about Configuration Mode in Vault Help. |
SDK_ERROR |
An error caused by the Vault Java SDK, generally a low-level trigger in the Vault platform. This could be a trigger that is part of the core application or custom trigger in a specific Vault. For more information about this error, check logs at the Admin -> Logs -> Vault Java SDK Logs area. |
Errors - EDC
Example: Outer vs. Inner responseStatus - Single subject add, fails...
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "FAILURE",
"errorMessage": "[Study Country] with name [Germany] not found"
"study_country": "Germany",
"site": "101",
"subject": "101-021",
}
]
}
Example: Outer vs Inner responseStatus - Add event groups, one succeeds, one fails...
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egIRT_INFO",
"eventgroup_sequence": 1
},
{
"responseStatus": "FAILURE",
"errorMessage": "[Subject] with name [101-021] not found"
"study_country": "United States",
"site": "101",
"subject": "101-021",
"eventgroup_name": "egIRT_INFO"
}
]
}
CDMS API requests for pushing data into the system allow for multiple actions in a single call. As such, a status per entry is provided in the responses. This is true even if the call only performs a single action.
Example: Incorrect study_name for Create Subject / Casebook
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "FAILURE",
"errorMessage": "[Study] with name [ABCP-2022-01_DEV2] not found",
"study_country": "United States",
"site": "101"
}
]
}
Example: Using Set Event Date on a Frozen Subject
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "FAILURE",
"errorMessage": "Subject is frozen",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "enrollment",
"eventgroup_sequence": 1,
"event_name": "enrollment_visit",
"event_sequence": 1
}
]
}
The table below lists common errors for EDC APIs:
| Area | Error | Description |
|---|---|---|
| Study | [Study] with name [study_name] not found | Vault couldn't find a Study with the Name provided in study_name. |
| Study | [Study Country] with name [study_country] not found | Vault couldn't find a Study Country with the Name provided in study_country. |
| Study | Study Country is provided, but Study Name is not | To use the study_country parameter, you must provide a value for the study_name parameter. |
| Study | [Study Site] with name [site] not found | Vault couldn't find a Site with the Site Number (site__v.name__v) provided in site. |
| Study | Site is provided, but Study Country is not | To use the site parameter, you must provide values for the study_name and study_country parameters. |
| Study | [Subject] with name [subject] not found | Vault couldn't find a Subject with the Name (Subject ID) provided in subject. |
| Study | [Subject] with name [subject] exists | A Subject with the Name (Subject ID) provided in subject already exists. |
| Study | Study is locked | The Study is locked, and so this action is not allowed. |
| Study | Site is locked | The Site is locked, and so this action is not allowed. |
| Study | Subject is provided, but Site and Study Country are not | To use the subject parameter, you must provide values for study_name, study_country, and study_site. |
| Study | Subject is frozen | The Subject is frozen, and so the action isn't allowed. |
| Study | Subject is locked | The Subject is locked, and so the action isn't allowed. |
| Study | [Event Definition] with [event_name] not found | Vault couldn't find an Event Definition with the Name provided in event_name. |
| Study | [Event Group Definition] with [eventgroup_name] not found | Vault couldn't find an Event Group Definition with the Name provided in eventgroup_name. |
| Study | [Form Definition] with name [form_name] not found | Vault couldn't find a Form Definition with the Name provided in form_name. |
| Study | [Item Group Definition] with name [itemgroup_name] not found | Vault couldn't find an Item Group Definition with the Name provided in itemgroup_name. |
| Study | [Item Definition] with name [item_name] not found | Vault couldn't find an Item Definition with the Name provided in item_name. |
| Study | Change reason is required | You didn't provide a value for change_reason, which is required for this API. |
| Study | Change reason too long | The change_reason you provided exceeds the maximum character limit. |
| Study | Country [country] does not exist | The country you provided is invalid. |
| Study | Event Date is frozen | The Event Date is frozen because either the Subject or Event is frozen, and so this action is not allowed. |
| Study | Event Date is locked | The Event Date is locked because either the Subject or Event is locked, and so this action is not allowed. |
| Study | Form is already submitted | The Form is already in the Submitted status, and you can't submit a Form that is already submitted, marked as intentionally left blank, or signed. |
| Study | Form is frozen | The Form is frozen, and so this action is not allowed. |
| Study | Form is locked | The Form is locked, and so this action is not allowed. |
| Study | Form is not submitted | The Form is not in the Submitted status, and so you can't use the Reopen Submitted Forms API to move it to the In Progress status. |
| Study | Item is locked | The Item is locked, and so this action is not allowed. |
| Study | Item value is not in correct format for setting the item | The value you provided isn't in the correct format for the Item. |
| Study | Items on submitted forms cannot be edited | The Item is on a Form that is in the Submitted status, and so you can't edit the Item. Use the Reopen Submitted Forms API to return the Form to the In Progress status. |
| Study | Unique event/item cannot be found with the specified keys` | Vault couldn't find the Event Date or Item for a repeating Event Group, Form, or Item Group with the matching sequence_number. |
| Study | Unique item cannot be found with the specified keys | Vault couldn't find the Form for this Item. |
| Queries | Query ID not found | Vault couldn't find a Query with the id provided. |
| Queries | Query is already in the Closed status | You can't answer or close a Query that is already in the Closed status. |
| Queries | Query is already in the Reopened status | You can't reopen a Query that is already in the Reopened status. |
| Queries | Query not in Closed status | You can't reopen a Query that isn't in the Closed status. |
| Queries | Query ID not found | Vault couldn't find a Query with the ID you provided in the id parameter. For this error, Vault returns SUCCESS but with an empty query list. |
| Queries | Derived field cannot be set | You can't set the value for an Item that is populated by a Set Item Value rule (derived item). |
| Queries | Message is required | The message parameter is required for this API. |
| Queries | Message is too long | The message provided exceeds the maximum character limit. |
| Queries | Unique query cannot be found with the specified keys | The Query is on an Event Date within a repeating Event Group or an Item on a repeating Form or Item Group, and Vault couldn't find the sequence with the matching sequence_number. Otherwise, Vault couldn't find the Query because the provided definitions were incorrect. |
| Jobs | Unsupported value provided for [Job Type] parameter, valid types are [event_progress_listing_v, subject_progress_listingv, form_progress_listingv, data_and_definition_exportv, study_data_extractv, core_listingv, query_detail_listing_v] | The value you provided for the job_type parameter was invalid. Valid job types can be found at: Job Summary / Types |
| Jobs | A job of the same type is already running | You can only have one in progress Study Data Extract job at any given time. If you receive this error, wait until the current job finishes before starting a new job. |
| Jobs | [Job] with [job_id] not found | Vault did not find a job with the provided job_id. |
| Jobs | [Job] with status [status] is not cancellable | You can only cancel jobs that are in the In Progress status. |
| Jobs | [Job] with status [status] is not able to return a log file | Vault can only return the log file when the job is no longer in the In Progress status. |
| Jobs | [Job] with status [status] is not able to return an export file | Job output files aren't available until the job is in the Completed status. |
| Jobs | [File Name] with name [...value...] invalid. File names may only include the characters a-z, A-Z, 0-9, -, _ and no double underscores | The file name you provided in file_name contained an invalid character. Provide a new value for file_name that only uses a-z, A-Z, 0-9, dashes (-), and underscores (_), and does not use spaces or double underscores (__). |
| Jobs | [File Name] should be entered without the .zip extension | The file name you provided in file_name included the extension (.zip). Remove the extension from file_name. |
| Jobs | [External Connection with name [external_connection] not found | Vault could not locate an External Connection with the Name that you provided in the external_connection parameter. |
| Jobs | [Forms] with name [forms] not found | Vault could not find a Form Definition with the Name you provided in the forms parameter. |
| Jobs | [forms] is a required parameter | The all_forms parameter was set to false, but you didn't include the forms parameter to specify a list of Forms. |
| Jobs | [all_forms] or [forms] are required | You must provide the all_forms parameter, and then either set it to true or provide a list of Forms in the forms parameter. |
| Jobs | [Export File Type] with [export_file_type] not found | The export_file_type you provided does not match one of the accepted values for the parameter: "CSV" or "SAS with XPT and CSV". |
| Jobs | SDE Version is invalid | The version of the Study Data Extract job you requested in the version parameter is invalid or unavailable. The available versions are ... (versions listed) |
| Medical Coding | Coding Request not found | The coding_request parameter is invalid or does not match an existing Code Request record. |
| Medical Coding | Coding Request is null | The coding_request parameter is required. |
| Medical Coding | Coding Request is blank | The coding_request parameter must have a value. |
| Subject / Casebook | Casebook cannot be added. [Study Site] with name [value] does not have an active casebook version. | The Site you are attempting to create a Casebook within does not have an Active Casebook Version assigned. |
| Users | Language [language] does not exist | The language you provided does not exist in Vault or is otherwise invalid. |
| Users | Last name must be no greater than 100 characters | The last_name provided exceeds the maximum character limit of 100 characters. |
| Users | Duplicate | A user with this user_name already exists. |
| Users | Locale [locale] does not exist | The locale you provided does not exist in Vault or is otherwise invalid. |
| Users | Email needs to be of valid format | The email you provided isn't in a valid format. |
| General | Date passed was empty or invalid format. Must use YYY-MM-DD. | You passed an invalid parameter for the date, meaning that it was empty, not ISO format, or otherwise invalid. |
| General | Last Modified Date must have the following format: yyyy-MM-dd'T'HH:mm:ss'Z' | You must use the yyyy-MM-dd'T'HH:mm:ss'Z' format for the last_modified_date parameter. |
| General | No permission for this action | You don't have the permissions required to use this API. |
| General | Error writing to destination directory, please check your FTP connection and directory permissions. | Vault encountered an error when attempting to send the output file to the FTP connection specified in the external_connection parameter. |
| General | No restricted data permission | You are attempting to request restricted data, but you don't have the Restricted Data Access permission. Update include_restricted_data to false. |
| General | The allowed maximum value for [limit] parameter is: 1000 | The maximum allowed value for the limit is 1000. Enter a number lower than 1000. |
| General | Expecting integer value for parameter [limit] but received [1a] | The limit operator requires a positive integer. |
| General | Expecting integer value for parameter [offset] but received [1a] | The offset operator requires a positive integer. |
| General | No results found using the resource_locator [offset] | A different user than the one who made the original request attempted to use the resource_locator (next_page/previous_page URLs) to paginate. The resource_locator request must be made by the same user who made the original request. |
Authentication
Authenticate your account using one of the methods outlined below. The response returns a session ID that you then use in subsequent API calls, inside the Authorization HTTP request header. Session IDs time out after a period of inactivity. The period varies by Vault.
User Name & Password
Request
curl -X POST -H "Content-Type: application/x-www-form-urlencoded" \
-d "username={username}&password={password}" \
"https://my-vault.veevavault.com/api/v24.1/auth"
Response
{
"responseStatus": "SUCCESS",
"sessionId": "802E62F765575BEB70642BE7A822A419F48B41312ECCAF4767D8DD956873DEE90D677F053A5DAB00B37E2C6B42FA6B15FCE6147C6120F56A638D911EBDFA007B",
"userId": 92677,
"vaultIds": [
{
"id": 1004329,
"name": "My Vault",
"url": "https://my-vault.veevavault.com/api"
},
{
"id": 1004330,
"name": "My Vault 2",
"url": "https://my-vault2.veevavault.com/api"
}
],
"vaultId": 1004329
}
Authenticate your account using your Vault username and password to obtain a Vault Session ID.
Endpoint
POST https://{vault_subdomain}/api/{version}/auth
Headers
| Name | Description |
|---|---|
Content-Type |
multipart/form-data or application/x-www-form-urlencoded |
Accept |
application/json (default) or application/xml |
URI Path Parameters
| Name | Description |
|---|---|
vault_subdomain |
The DNS of the Vault for which you want to generate a session. If this domain is invalid or inactive, Vault executes logic to authenticate the user to their most relevant available Vault. The term invalid applies to any Vault where the requesting user is unable to authenticate. Learn more about default Vaults. |
version |
The Vault REST API version. Your authentication version does not need to match the version in subsequent calls. For example, you can authenticate with v22.2 and run your integrations with v22.3. |
Body Parameters
| Name | Description |
|---|---|
username |
The username of your Vault account |
password |
The password of your Vault account |
vaultDNS |
Optional The DNS of the Vault for which you want to generate a session. If omitted, generates a session for the user’s default Vault. |
Response Details
On SUCCESS, this request returns a valid sessionId for any Vault DNS where the user has access.
The Vault DNS for the returned session is calculated in the following order:
- Generates a session for the DNS in the optional
vaultDNSbody parameter. If thisvaultDNSis invalid, generates a session for the user’s most relevant available Vault:
- Generates a session for the Vault where the user last logged in
- If the user has never logged in, or if the last logged-in Vault is inactive, generates a session for the oldest active Vault where that user is a member
- If the user is not a member of any active Vaults, the user cannot authenticate, and the API returns
FAILURE
- If the optional
vaultDNSbody parameter is omitted, generates a session for the DNS specified in thevaultDNSURI parameter. - If this
vaultDNSis invalid, generates a session for the user’s most relevant available Vault:
- Generates a session for the Vault where the user last logged in
- If the user has never logged in, or if the last logged-in Vault is inactive, generates a session for the oldest active Vault where that user is a member
- If the user is not a member of any active Vaults, the user cannot authenticate, and the API returns
FAILURE
An invalid DNS is any DNS that the specified user cannot access, for example, if the DNS does not exist, if the user does not exist in that DNS, or if all Vaults in that DNS are inactive.
It is best practice to inspect the response, compare the desired Vault ID with the list of returned vaultIds, and confirm the DNS matches the expected login. This API only returns FAILURE if it is unable to return a valid sessionIdfor any Vault the user can access.
Basic Authorization
| Name | Description |
|---|---|
Authorization |
{sessionId} |
Alternatively, you can use Salesforce™ or OAuth2/OIDC Delegated Requests.
The Vault API also accepts Vault session IDs as bearer tokens. Include the Bearer keyword to send Vault session IDs as bearer tokens.
Bearer Token Authorization
| Name | Description |
|---|---|
Authorization |
Bearer {sessionId} |
OAuth 2.0 / OpenID Connect
Request
$ curl -X POST \
-H "Authorization: Bearer 1C29326C3DF" \
-H "Host: Bearer 1C29326C3DF" \
"https://my-vault.veevavault.com/auth/oauth/session/_9ad0a091-cbd6-4c59-ab5a-d4f2870f218c"
Response
{
"responseStatus": "SUCCESS",
"sessionId": "802E62F765575BEB70642BE7A822A419F48B41312ECCAF4767D8DD956873DEE90D677F053A5DAB00B37E2C6B42FA6B15FCE6147C6120F56A638D911EBDFA007B",
"userId": 92677,
"vaultIds": [
{
"id": 1004329,
"name": "My Vault",
"url": "https://my-vault.veevavault.com/api"
},
{
"id": 1004330,
"name": "My Vault 2",
"url": "https://my-vault2.veevavault.com/api"
}
],
"vaultId": 1004329
}
Authenticate your account using OAuth 2.0 / Open ID Connect token to obtain a Vault Session ID. Learn more about OAuth 2.0 / Open ID Connect in Vault Help.
When requesting a sessionId, Vault allows the ability for Oauth2/OIDC client applications to pass the client_id with the request. Vault uses this client_id when talking with the introspection endpoint at the authorization server to validate that the access_token presented by the application is valid. More information on client_id found in a previous section.
Endpoint
POST https://login.veevavault.com/auth/oauth/session/{oath_oidc_profile_id}
Headers
| Name | Description |
|---|---|
Authorization |
Bearer {access_token} |
Accept |
application/json (default) |
URI Path Parameters
| Name | Description |
|---|---|
oath_oidc_profile_id |
The ID of your OAuth2.0 / Open ID Connect profile |
Body Parameters
| Name | Description |
|---|---|
vaultDNS |
Optional The DNS of the Vault for which you want to generate a session. If omitted, the session is generated for user’s default Vault. |
client_id |
Optional The ID of the client application at the Authorization server |
Authentication Type Discovery
Request
$ curl -X GET \
-H "Accept: application/json" \
"https://login.veevavault.com/auth/discovery?username=meganmurray@veepharm.com&client_id=VaultCheckOut"
Response: Password User
{
"responseStatus": "SUCCESS",
"errors": [],
"data": {
"auth_type": "password"
}
}
Response: SSO User
{
"responseStatus": "SUCCESS",
"data": {
"auth_type": "sso",
"auth_profiles": [
{
"id": "_9ad0a091-cbd6-4z59-ab5a-d4f35789918c",
"label": "VeePharm",
"description": "",
"vault_session_endpoint": "https://veepharm.com/auth/oauth/session/_9ad0a091-cbd6-4z59-ab5a-d4f35789918c",
"use_adal": false,
"as_client_id":"34524523452345234523452345098098234",
"as_metadata": {
"issuer": "https://veevaintrospection.com/oauth2/asdf123",
"authorization_endpoint": "https://veevintrospection.com/oauth2/asdf123/v1/authorize",
"token_endpoint": "https://veevaintrospection.com/oauth2/asdf123/v1/token",
"registration_endpoint": "https://veevaintrospection.com/oauth2/v1/clients",
"jwks_uri": "https://veevaintrospection.com/oauth2/asdf123/v1/keys",
"response_types_supported": [
"code",
"token",
"code token"
],
"response_modes_supported": [
"query"
],
"introspection_endpoint": "https://veevatintrospection.com/oauth2/asdf1234/v1/introspect",
"introspection_endpoint_auth_methods_supported": [
"client_secret_basic",
],
"revocation_endpoint": "https://veevaintrospection.com/oauth2/asdf123/v1/revoke",
"revocation_endpoint_auth_methods_supported": [
"client_secret_basic",
],
"end_session_endpoint": "https://veevaintrospection.com/oauth2/asdf123/v1/logout"
}
}
]
}
}
Discover the user's authentication type. With this API, applications can dynamically adjust the login requirements per user, and support either username/password or OAuth2.0 / OpenID Connect authentication schemes.
Endpoint
POST https://login.veevavault.com/auth/discovery
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Query String Parameters
| Name | Description |
|---|---|
username |
The user’s Vault username |
client_id |
Optional The user's mapped Authorization Server client_id. This only applies the SSO auth_type. |
Response Details
The response specifies the user’s authentication type (auth_type):
password: The user is configured with a username and password.sso: The user is configured with an SSO Security Policy and at least one SSO profile.
If the user’s authentication type is sso, the response specifies the user’s authentication profiles (auth_profiles). If the user’s Security Policy is associated with:
- An OAuth 2.0 / OpenID Connect profile, the response will also contain the Authentication Server metadata (
as_metadata). - A SAML profile, the
auth_profilesarray will be empty.
If the Authorization Server Provider is set to use ADFS, the use_adal field will appear in the response as true. If the Authorization Server Provider is set to anything else, this field is false.
If the user provides a client_id and Client Application client ID mapping is defined on the OAuth 2.0 / OpenID Connect profile, the as_client_id field will appear in the response with the Authorization Server client ID value. If there is no defined mapping for the specified client_id, Vault will not include the as_client_id field in the response. Learn about Client ID Mapping in Vault Help.
Studies
Clinical Data uses the Study as the main organization of data for a trial. Components that exist under a Study include Study Countries, Sites, Subjects (also known as Casebooks), Queries, Medical Coding Requests, Study Jobs, and Form data.
Retrieve Studies
Request
curl -L -X GET 'https://myvault.veevavault.com/api/v24.1/app/cdm/studies' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"studies": [
{
"study": "Labrinone_DEV1",
"study_name": "Labrinone_DEV1",
"study_label": "Labrinone_DEV1",
"external_id": "Labrinone",
"study_phase": "phase_ii__v",
"study_status": "planning__v",
"locked": true,
"last_locked_date": "2023-06-02T12:00:00Z",
"connect_to_vault_ctms": true,
"organization": "ABC Pharma",
"environment_type": "development__v",
"build_number": 14,
"casebook_versions": [
{
"casebook_version": 1,
"version_name": "Initial Version",
"external_id": "1",
"previous_version_name": null,
"description": null,
"change_reason": null,
"casebook_status": "in_progress__v",
"created_by": "Eric Emerton",
"created_date": "2023-02-12T22:32:21Z",
"last_modified_date": "2023-03-04T14:16:20Z"
}
]
},
{
"study_name": "ABCP-2022-01_DEV1",
"external_id": "ABCP-2022-01",
"study_phase": "phase_i__v",
"study_status": "execution__v",
"locked": false,
"last_locked_date": null,
"connect_to_vault_ctms": false,
"organization": "ABC Pharma",
"environment_type": "development__v",
"build_number": 43,
"casebook_versions": [
{
"casebook_version": 2,
"version_name": "Version2",
"external_id": "Version2",
"previous_version_name": "Initial Version",
"description": "Casebook Definition with changes for the ABCP-2022-01_DEV1 study" ,
"change_reason": "Layout changes",
"casebook_status": "in_progress__v",
"created_by": "Maisha Muddaththir",
"created_date": "2023-06-28T14:26:34Z",
"last_modified_date": "2023-06-28T14:26:34Z"
},
{
"casebook_version": 1,
"version_name": "Initial Version",
"external_id": "Initial Version",
"previous_version_name": null,
"description": "Initial casebook definition version for the ABCP-2022-01_DEV1 study" ,
"change_reason": null,
"casebook_status": "published__v",
"created_by": "Maisha Muddaththir",
"created_date": "2023-06-09T13:26:34Z",
"last_modified_date": "2023-06-09T13:26:34Z"
}
]
}
]
}
Notes
- Used to retrieve the list of Studies in the Vault.
- The
casebook_versionsarray within eachstudiesentry indicates design casebook versions for the Study. - Versions are assigned at add Subject per the active version at that Site. Later, during amendments, a Subject / Site / entire Study can transition from one version to the next.
- ONLY the Studies the user has permissions and are indicated as trained appear in responses from the API. Contact an administrator on the Vault to have an override set regarding training.
- A coming release of the API will allow for usage of either
study(match on Study label) orstudy_name. For clarity on this change, this endpoint (at 23R3) includes all four of these values:study_name,study_label,study_external_id, and thestudy(always matchesstudy_label) - At 23R3 - the
study_phaseandstudy_statuswere changed to picklist name where it used to be picklist label. (picklist name is the Vault standard)
Endpoint
GET https://my-vault.veevavault.com/api/v24.1/app/cdm/studies
Required Permissions
The following permissions are required to use the Retrieve Studies API:
- API Access
Both the CDMS API Read Only and CDMS API Read Write roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Studies that you have permission to view.
| Array | Name | Description |
|---|---|---|
studies |
study |
This value is always the same as study_label |
studies |
study_name |
Name of the Study. Although very rare, this may differ from the Study label seen on screen |
studies |
study_label |
Label of the Study. This is the Study identification through the UI, reports, exports. It can - on rare occasion - differ from study_name (e.g. relabel). |
studies |
external_id |
External ID field value from the Study record |
studies |
study_phase |
Study Phase picklist name (not label) from the Study record |
studies |
study_status |
Study Status picklist name (not label) from the Study record |
studies |
locked |
Whether the Study is currently locked, or not (true/false) |
studies |
last_locked_date |
The date/time of the last locking of the Study |
studies |
connect_to_vault_ctms |
Whether the Study is currently connected to a Vault CTMS (true/false) |
studies |
organization |
Name of the organization in the Vault that the Study is grouped under |
studies |
environment_type |
The type of environment for the Study - picklist name (not label) |
studies |
build_number |
For the Study its build number from Study design. Design and other information can be retrieved in further detail via Retrieve Study Masters when using the main development Vault |
studies |
casebook_versions |
For each Casebook Version associated with the Study, Vault returns the following as an array:
|
studies/casebook_versions |
casebook_version |
The version number (integer) of the casebook definition |
studies/casebook_versions |
version_name |
The version name of the casebook definition |
studies/casebook_versions |
external_id |
The external ID of the casebook definition |
studies/casebook_versions |
previous_version_name |
Not in use, return is null |
studies/casebook_versions |
description |
The descripition of the casebook definition |
studies/casebook_versions |
change_reason |
A reason for the casebook version (if set) |
studies/casebook_versions |
casebook_status |
The status of the casebook version, e.g. published__v, validated__v, etc. (Vault picklist values) |
studies/casebook_versions |
created_by |
The name of the user that added (or deployed to) the casebook version |
studies/casebook_versions |
created_date |
The creation date/time of the add (or deployment) |
studies/casebook_versions |
last_modified_by |
The name of the user that last modified the casebook definition |
Study Countries
Clinical Data uses a Study Country to group together Sites.
Retrieve Study Countries
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/sites?study_name=ABCP-2022-01_DEV1' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 3,
"total": 3
},
"study_countries": [
{
"study_country": "United States",
"country": "United States",
"country_abbreviation": "USA",
"created_by": "Eric Emerton",
"created_date": "2022-05-23T17:17:24Z",
"last_modified_date": "2022-05-23T17:17:24Z"
},
{
"study_country": "Austria",
"country": "Austria",
"country_abbreviation": "AUT",
"created_by": "Eric Emerton",
"created_date": "2022-05-23T17:22:45Z",
"last_modified_date": "2022-05-23T17:22:45Z"
},
{
"study_country": "Canada",
"country": "Canada",
"country_abbreviation": "CAN",
"created_by": "Eric Emerton",
"created_date": "2022-05-23T17:22:53Z",
"last_modified_date": "2022-05-23T17:22:53Z"
}
]
}
Notes
- Used to get the Study Countries of a Study.
- Each are activated into a Study from the Vault master country list (
country__vobject). - Study Country information is also returned in most endpoint responses.
Endpoint
GET /api/{version}/app/cdm/studycountries
Required Permissions
The following permissions are required to use the Retrieve Study Countries API:
- API Access
Vault only returns Study Countries to which the logged in user has access, even if more Study Countries exist.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query Parameters
Include the following parameters to filter the results:
| Name | Description |
|---|---|
study_name |
The Name (Study Number) of the Study (study__v.name__v) that you want to retrieve the study countries for, returned as study_name by the Retrieve Studies API. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer). If omitted, the default is 1. For example, if the limit is set to "100", and there are 150 possible records, use "101" as the offset to show results 101 through 150. |
Response Details
Vault returns the following details about each Study Country:
| Array | Name | Description |
|---|---|---|
study_countries |
study_country |
The Name of the Study Country (study_country__v.name__v) |
study_countries |
country |
The Name of the Country for the Study Country (study_country__vr.country__v.name__v) |
study_countries |
abbreviation |
The Abbreviation of the Country for the Study Country (study_country__vr.country__v.abbreviation__v) |
study_countries |
created_by |
The User Name of the User who created the Study Country (study_country__v.created_by__v) |
study_countries |
created_date |
The Created Date for the Study Country (study_country__v.created_date__v) |
study_countries |
last_modified_by |
The Last Modified Date for the Study Country (study_country__v.last_modified_date__v) |
If the Study does not yet contain any Study Countries, Vault returns an empty array (example to the right)
{
"responseStatus": "SUCCESS",
"study_countries": []
}
Sites
A Site in Clinical Data represents a single clinical research location. A given Study may have any number of Sites, under which are the Subjects of the Site. Properties for a Site include status, assigned Casebook version for newly added Subjects, principal investigator, Review Plans, and the Study Country to which they belong. Learn more about Sites in Clinical Data Help.
Retrieve Sites
Request - All sites in the study
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/sites?study_name=ABCP-2022-01_DEV1' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - All sites in the study
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 4,
"total": 4
},
"sites": [
{
"site": "101",
"site_name": "Cary Hospital",
"site_status": "active__v",
"site_closeout_status": "",
"study_country": "United States",
"principal_investigator": "Linda Johnson",
"casebook_version": 1,
"timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)"
},
{
"site": "102",
"site_name": "Raleigh Hospital",
"site_status": "active__v",
"site_closeout_status": "",
"study_country": "United States",
"principal_investigator": "Mary Peters",
"casebook_version": 1,
"timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)"
},
{
"site": "201",
"site_name": "Montreal General",
"site_status": "active__v",
"site_closeout_status": "",
"study_country": "Canada",
"principal_investigator": "Jim Jones",
"casebook_version": 1,
"timezone": "(GMT-06:00) Central Standard Time (America/Chicago)"
},
{
"site": "202",
"site_name": "No Active CB Version",
"site_status": "active__v",
"site_closeout_status": "",
"study_country": "United States",
"principal_investigator": "Mary Smith",
"casebook_version": null,
"timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)"
}
]
}
Request - No sites in study yet
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/sites?study_name=ABCP-2022-01_DEV1' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - No sites in study yet
{
"responseStatus": "SUCCESS",
"sites": []
}
Notes
- Used to get the Sites of a Study
- Optionally, get the Sites of only one Study Country
- Casebook version (i.e. what new Subjects added to the site will receive) is returned with other attributes
Endpoint
GET https://my-vault.veevavault.com/api/v24.1/app/cdm/sites
Required Permissions
The following permissions are required to use the Retrieve Sites API:
- API Access
- View Study Sites
Both the CDMS API Read Only and CDMS API Read Write roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study. |
study_country |
Optional Name of Study Country to filter on. If omitted, Vault returns all Sites. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
In the RESPONSE, Vault returns the following details about each Site:
| Array | Name | Description |
|---|---|---|
sites |
site |
Site Number of the Site e.g. 101, 102, 201, or however the study defines Site numbering |
sites |
site_name |
Name of the Site. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
sites |
site_status |
Status of the Site. This is one of the following possible statuses:
|
sites |
site_closeout_status |
Closeout Status of the Site. If the closeout process hasn't started for the Site, this value is blank ("").This is one of the following possible statuses:
|
sites |
study_country |
Name of the Study Country of the Site |
sites |
principal_investigator |
The Principal Investigator assigned to the Site. The PI's name source changes depending on the feature flag entitled “Enable Person and Just in Time VeevaID User Creation”. If he flag is on, the name comes from the person__sys field, and if the flag is off, it comes from the person__v field. |
sites |
casebook_version |
Casebook Version of the Site. This is the version of the Study scheduled of Events / Forms newly created Subjects will receive. Some Sites might be on different versions of the design (all Sites do not have to be on the same version at once). This important version is assigned by a user via the UI, at Tools -> EDC Tools. Learn more about Sites in Clinical Data Help |
sites |
timezone |
The Timezone of the Site (site__v.timezone__v) |
Subjects / Casebooks
Clinical Data uses two objects to manage participants in a Study: Subject and Casebook. For the purposes of the EDC API, these are synonyms. A Subject record represents an individual participating in a Study. This object is where Vault tracks the Subject's status as they progress through the Study. A Subject's Casebook object record contains all other information, including visits and form data, for that Subject in the given Study.
Retrieve Subjects
Request - By Study Context
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects?study_name=ABCP-2022-01_DEV1' \
-H 'Authorization: {SESSION_ID}'
Response - By Study Context
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 7,
"total": 7
},
"subjects": [
{
"id": "OPP00000000I001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "SCR-0006",
"status": "pre_screen__v",
"created_date": "2022-02-04T20:11:46Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-02-04T20:11:46Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000000I001&tab_name=data_entry__v"
},
{
"id": "OPP00000000H001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "SCR-0005",
"status": "pre_screen__v",
"created_date": "2022-02-04T20:11:32Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-02-04T20:11:32Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000000H001&tab_name=data_entry__v"
},
{
"id": "OPP00000000A001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "102",
"subject": "102-001",
"status": "in_screening__v",
"created_date": "2021-09-21T23:12:55Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2021-09-21T23:13:42Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000000A001&tab_name=data_entry__v"
},
{
"id": "OPP000000009002",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "101-003",
"status": "in_screening__v",
"created_date": "2021-09-21T23:08:00Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2021-09-21T23:12:44Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000009002&tab_name=data_entry__v"
},
{
"id": "OPP000000009001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "101-002",
"status": "in_screening__v",
"created_date": "2021-09-21T23:07:07Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2021-09-21T23:07:50Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000009001&tab_name=data_entry__v"
},
{
"id": "OPP000000000501",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"status": "enrolled__v",
"created_date": "2021-02-12T18:51:53Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2021-03-11T20:23:56Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000501&tab_name=data_entry__v"
},
{
"id": "OPP000000000502",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "103",
"subject": "103-001",
"status": "enrolled__v",
"created_date": "2020-02-12T18:51:53Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-08-11T20:23:56Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000502&tab_name=data_entry__v"
}
]
}
Request - By IDs (two specific)
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects?study_name=ABCP-2022-01_DEV1&id=OPP00000000I001,OPP00000000H001' \
-H 'Authorization: {SESSION_ID}'
Response - By IDs (two specific)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"subjects": [
{
"id": "OPP00000000I001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "SCR-0006",
"status": "pre_screen__v",
"created_date": "2022-02-04T20:11:46Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-02-04T20:11:46Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000000I001&tab_name=data_entry__v"
},
{
"id": "OPP00000000H001",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "101",
"subject": "SCR-0005",
"status": "pre_screen__v",
"created_date": "2022-02-04T20:11:32Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-02-04T20:11:32Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000000H001&tab_name=data_entry__v"
}
]
}
Request - By Multiple Sites
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects?study_name=ABCP-2022-01_DEV1&site=102,103' \
-H 'Authorization: {SESSION_ID}'
Response - By Multiple Sites
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 4,
"total": 4
},
"subjects": [
{
"id": "OPP00000001U002",
"study_name": "ABCP-2022-01_DEV1",
"site": "102",
"site_name": "Raleigh Hospital",
"study_country": "United States",
"subject": "102-001",
"status": "pre_screen__v",
"casebook_version": 1,
"created_date": "2022-07-07T16:40:24Z",
"created_by": "Eric Emerton",
"last_modified_date": "2022-07-07T16:40:25Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001U002&tab_name=data_entry__v"
},
{
"id": "OPP00000001I004",
"study_name": "ABCP-2022-01_DEV1",
"site": "102",
"site_name": "Raleigh Hospital",
"study_country": "United States",
"subject": "SCR-0001",
"status": "in_screening__v",
"casebook_version": 1,
"created_date": "2022-05-23T18:36:00Z",
"created_by": "Eric Emerton",
"screened_date": "2022-01-03",
"last_modified_date": "2022-07-05T13:04:00Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001I004&tab_name=data_entry__v"
},
{
"id": "OPP00000001Q003",
"study_name": "ABCP-2022-01_DEV1",
"site": "102",
"site_name": "Raleigh Hospital",
"study_country": "United States",
"subject": "SCR-0002",
"status": "complete__v",
"casebook_version": 1,
"created_date": "2022-06-22T20:53:42Z",
"created_by": "Eric Emerton",
"initial_consent_date": "2022-06-02",
"screened_date": "2022-06-01",
"screen_failed_date": "2022-06-02",
"enrolled_date": "2022-06-04",
"randomized_date": "2022-06-03",
"start_treatment_date": "2022-06-21",
"end_treatment_date": "2022-06-06",
"withdrawn_date": "2022-06-05",
"started_follow_up_date": "2022-06-28",
"lost_to_follow_up_date": "2022-06-30",
"end_study_date": "2022-06-07",
"last_modified_date": "2022-06-23T17:20:06Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001Q003&tab_name=data_entry__v"
},
{
"id": "OPP000000000502",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "103",
"subject": "103-001",
"status": "enrolled__v",
"created_date": "2022-02-12T18:51:53Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-08-11T20:23:56Z",
"casebook_version": 1,
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000502&tab_name=data_entry__v"
}
]
}
Request - Where Subjects have IXRS IDs
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects?study_name=ABCP-2022-01_DEV1&site=103' \
-H 'Authorization: {SESSION_ID}'
Response - Where Subjects have IXRS IDs
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"subjects": [
{
"id": "OPP000000000502",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "103",
"subject": "103-001",
"status": "enrolled__v",
"created_date": "2022-02-12T18:51:53Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-08-11T20:23:56Z",
"casebook_version": 1,
"ixrs_id": "1234-ABCDE-456",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000502&tab_name=data_entry__v"
},
{
"id": "OPP000000000503",
"study_name": "ABCP-2022-01_DEV1",
"study_country": "United States",
"site": "103",
"subject": "103-001",
"status": "enrolled__v",
"created_date": "2022-02-12T18:51:53Z",
"created_by": "Cordelia Hunter",
"last_modified_date": "2022-08-11T20:23:56Z",
"casebook_version": 1,
"ixrs_id": "1234-ABCDE-479",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000503&tab_name=data_entry__v"
}
]
}
Notes
- Used to get the Subjects of a Study
- The response returned includes additional dates for milestones of the Subject. These only appear when there is a date value, and are omitted from response when not set. Milestone dates are set by either study design configuration rules, or through the Set Subject Status endpoint.
- In addition to the response examples to the right, consider a Study with these Sites - United States (101, 102), Canada (201, 202), United Kingdom (301,302), and filtering examples:
| Style | Example | Results / Notes |
|---|---|---|
| Study Context | .../subjects?study_name={..} |
All subjects |
| Study Context | .../subjects?study_name={..}&study_country=Canada&site=101 |
Site 101 subjects |
| Study Context | .../subjects?study_name={..}&site=101 |
Site 101 subjects (with/without country, same) |
| Study Context | .../subjects?study_name={..}&site=101,102 |
Site 101 and Site 102 subjects |
| Study Context | .../subjects?study_name={..}&site=101,102,501 |
Site 101 and Site 102 subjects (same, 501 does not exist) |
| Study Context | .../subjects?study_name={..}&site=501,502 |
ERROR - Neither exists, error return (not empty array), that is, no valid sites |
| Study Context | .../subjects?study_name={..}&study_country=Canada&site=101,102 |
ERROR - Multi site search is not allowed when including a study country, even if both reside at that country |
| Study Context | .../subjects?study_name={..}&last_modified_date=2023-07-01T12:00:00Z |
Subjects modified since July 1st, 12:00 UTC |
| By ID | .../subjects?study_name={..}&id=OPP00000001V002 |
Just the one subject with matching Vault ID, assuming valid |
| By ID | .../subjects?study_name={..}&id=OPP00000001V002,OPP00000001V003 |
The two subjects, assuming both are valid Vault IDs |
| By ID | .../subjects?study_name={..}&id=OPP00000001V077,OPP00000001V088 |
Assuming neither exists, an empty JSON Array (not an error) |
Endpoint
GET https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects
Required Permissions
The following permissions are required to use the Retrieve Subjects API:
- API Access
- View Casebook
Both the CDMS API Read Only and CDMS API Read Write roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Query String Parameters (by Study Context)
| Name | Description |
|---|---|
study_name |
Name of the Study |
study_country |
Optional Name of the Study Country to filter on. If omitted, Vault returns all Subjects for the given Study. |
site |
Optional Name of the Sites to filter on (comma-separated). If omitted, Vault returns all Subjects for the Study. If multiple is provided, Study Country must by omitted, even if the multiple listed are all from the same Study Country |
last_modified_date |
Optional Filter to only Subjects modified after the specified datetime. Use the format: "yyyy-MM-ddTHH:mm:ssZ". If omitted, Vault retrieves all Subjects for the Study with respect to last modified date. |
Query String Parameters (by ID)
| Name | Description |
|---|---|
study_name |
Name of the Study |
id |
The Vault internal ID of the Subject, or multiple in a comma-separated list. The id value can be found from Retrieve Subjects. This parameter cannot be used in conjunction with Study Country or Site or last modified filters |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Subjects meeting your filter criteria. If there are no Subjects meeting your filter criteria, Vault returns an empty array.
| Array | Name | Description |
|---|---|---|
subjects |
study_name |
Name of the Study |
subjects |
id |
Vault internal ID for the Subject (will never change, whereas subject name might) |
subjects |
site |
Name of the Site assigned to the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subjects |
study_country |
Name of the Study Country assigned to the Subject |
subjects |
subject |
Subject ID (Name) of the Subject |
subjects |
status |
Subject Status assigned to the Subject. Common statuses include:
|
subjects |
created_date |
The datetime that the Subject was first created |
subjects |
created_by |
Name (Firstname Lastname) of the User who created the Subject |
subjects |
initial_consent_date* |
Initial Consent Date for the Subject (only present when a non-empty value) |
subjects |
screened_date* |
Screened Date for the Subject (only present when a non-empty value) |
subjects |
screen_failed_date* |
Screen Failed Date for the Subject (only present when a non-empty value) |
subjects |
enrolled_date* |
Enrolled Date for the Subject (only present when a non-empty value) |
subjects |
randomized_date* |
Randomized Date for the Subject (only present when a non-empty value) |
subjects |
start_treatment_date* |
Start Treatment Date for the Subject (only present when a non-empty value) |
subjects |
end_treatment_date* |
End Treatment Date for the Subject (only present when a non-empty value) |
subjects |
withdrawn_date* |
Withdrawn Date for the Subject (only present when a non-empty value) |
subjects |
started_follow_up_date* |
Started Follow Up Date for the Subject (only present when a non-empty value) |
subjects |
lost_to_follow_up_date* |
Lost to Follow Up Date for the Subject (only present when a non-empty value) |
subjects |
end_study_date* |
End Study Date for the Subject (only present when a non-empty value) |
subjects |
last_modified_date |
The datetime that the Subject was last updated |
subjects |
casebook_version |
The study design casebook version of the Subject, currently |
subjects |
ixrs_id |
An external ID, typically from IXRS/IRT upstream system. The value would have been set at creation of the Subject (only present when a non-empty value) |
subjects |
cdms_url |
Relative URL to the Subject, at the Data Entry tab |
Create Subjects (Casebooks)
Request - Two subjects created at once
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States"
"site": "101"
},
{
"study_country": "United States",
"site": "102"
}
]
}'
Response - Two subjects created at once
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0003",
"id": "OPP00000001V002",
"ixrs_id": "",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001V002&tab_name=data_entry__v"
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "102",
"subject": "SCR-0004",
"id": "OPP00000001V003",
"ixrs_id": "",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001V003&tab_name=data_entry__v"
}
]
}
Request - Two subjects attempted, with specific numbering, one fails
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States"
"site": "101" ,
"subject": "101-001"
},
{
"study_country": "Belgium",
"site": "103" ,
"subject": "103-001"
}
]
}'
Response - Two subjects attempted, with specific numbering, one fails
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"study_country": "United States"
"site": "101",
"subject": "101-001",
"id": "OPP00000001V005",
"ixrs_id": "",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001V005&tab_name=data_entry__v"
},
{
"responseStatus": "FAILURE",
"errorMessage": "[Study Country] with name [Belgium] not found"
"study_country": "Belgium"
"site": "103",
"subject": "103-001"
}
]
}
Request - Two subjects, with IXRS IDs
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States"
"site": "101" ,
"subject": "101-001",
"ixrs_id": "ABC-123123-56"
},
{
"study_country": "Belgium",
"site": "103" ,
"subject": "103-001",
"ixrs_id": "ABC-123123-78"
}
]
}'
Response - Two subjects, with IXRS IDs
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"study_country": "United States"
"site": "101",
"subject": "101-001",
"id": "OPP00000001V005",
"ixrs_id": "ABC-123123-56",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001V005&tab_name=data_entry__v"
},
{
"responseStatus": "SUCCESS",
"study_country": "United States"
"site": "101",
"subject": "101-001",
"id": "OPP00000001V006",
"ixrs_id": "ABC-123123-78",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP00000001V006&tab_name=data_entry__v"
}
]
}
Notes
- Used to add a Subject (also known as Casebook) into the Study.
- The
subjectparameter is optional. When omitted, the next auto screening number is assigned, which follows the pattern SCR-0001, SCR-0002, SCR-0003 (although configurable) within each Site. - Vault also creates the first Event Group for the Subject and the first Event (visit) within that group. The action does not set any Event dates.
- To set the Event's date, you must use Set Event Date. Learn more about Casebooks in Clinical Data Help.
- The limit for actions in one request is 100.
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects
Required Permissions
The following permissions are required to use the Create Subjects (Casebooks) API:
- API Access
- Add Casebook
- View Casebook
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study to contain the Subject/Casebook (study__v.name__v) |
|
study_country |
subjects |
Name of the Study Country for the site of Subject/Casebook |
site |
subjects |
Name of the Site for the Subject/Casebook. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
subjects |
Optional The Subject ID (subject__v.name__v) on create of the Subject. If you don’t include a subject, Vault creates a screening number for the Subject. (e.g. SCR-000X, depending on study design). |
ixrs_id |
subjects |
Optional The value in an IRT/IXRS system who adds the Subject, i.e. the ID it identifies the Subject with. |
Set Subject Status
Request
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/setstatus' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States",
"site": "101",
"subject": "101-0010",
"subject_status": "randomized__v",
"date": "2020-03-03"
}
]
}'
Response
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"study_country": "United States"
"site": "101",
"subject": "101-0010",
"subject_status": "randomized__v"
}
]
}
Notes
- Used to set the status of a Study
- Often done as a 2nd step, right after adding of a new Subject to advance past Pre-Screening default status to Screening, Enrolled, or other.
- That
subject_statusvalue must be the API name, not the label (e.g.enrolled__v). - The set of a milestone date, i.e., the date the Subject took on that status is required.
- The action is the equivalent of a Studio design rule, which can also set the milestone date and change status for the Subject.
- Refer to the table below for order of precedence of Subject statuses, when setting or removing.
- Currently, the set of multiple Subjects, or multiple statuses for one Subject (in one API call) is not allowed. The format used is still of batch style like other endpoints, but multiple in one request is future release.
- A Subject does not have to go through each Subject status. Study design requirements dictate which statuses a Subject transitions through.
- If the Subject is already in the Status specified in your request, Vault does not change the Status or update the status's milestone date.
- If the Subject's current status is more advanced than the one specified in your request, Vault only moves the Subject into the lower status if that status is the previous in precedence. Otherwise, the request fails.
- You can undo any changes made with this API using Unset Subject Status.
- The various status / milestone dates are found in Retrieve Subjects.
| Order | Subject Status | API Value | Return in Retrieve Subjects |
|---|---|---|---|
| 1 | Pre Screen | ||
| 2 | Consented | consented__v |
initial_consent_date |
| 3 | In Screening | in_screening__v |
screened_date |
| 4 | Screen Failure | screen_failure__v |
screen_failed_date |
| 5 | Enrolled | enrolled__v |
enrolled_date |
| 6 | Randomized | randomized__v |
randomized_date |
| 7 | Started Treatment | started_treatment__v |
started_treatment_date |
| 8 | End Of Treatment | end_of_treatment__v |
end_of_treatment_date |
| 9 | Withdrawn | withdrawn__v |
withdrawn_date |
| 10 | Started Follow Up | started_follow_up__v |
started_follow_up_date |
| 11 | Lost to Follow Up | lost_to_follow_up__v |
lost_to_follow_up_date |
| 12 | Complete | complete__v |
end_of_study_date |
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/setstatus
Required Permissions
The following permissions are required to use the Set Subject Status API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
subjects |
Name of the Study Country of the Subject |
site |
subjects |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
subjects |
Name of the Subject (ID / number) |
subject_status |
subjects |
The status to set for the Subject. As of this release only one entry in the subjects array is allowed per request. |
date |
subjects |
The date to set for the status milestone for the Subject. Use yyyy-MM-dd format for the status milestone date. |
Unset Subject Status
Request
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/unsetstatus' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"site": "101",
"study_country": "United States",
"subject": "101-0010",
"subject_status": "enrolled__v"
}
]
}'
Response
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"study_country": "United States"
"site": "101",
"subject": "101-0010",
"subject_status": "in_screening__v"
}
]
}
Notes
- Used to unset the status of a Subject.
- The action will also remove the milestone date previously set.
- As an example, suppose a Subject has progressed In Screening -> Enrolled -> Randomized. If the endpoint is used to unset Enrolled, this results in BOTH the Enrolled and Randomized milestone dates being removed.
- See the section above for an order of precedence of Subject statuses.
- Currently, the unset of multiple Subjects, or multiple for one Subject (in one API call) is not allowed. The format used is still of batch style like other endpoints, but multiple in one request is future release.
- The response includes the Subject status that was in the request body, not the current status of the Subject after the action.
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/unsetstatus
Required Permissions
The following permissions are required to use the Unset Subject Status API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
subjects |
Name of the Study Country of the Subject |
site |
subjects |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
subjects |
Name of the Subject (ID / number) |
subject_status |
subjects |
The status to unset for the Subject. As of this release only one entry in the subjects array is allowed per request. The Subject will remain in the highest possible status. See the list above. |
Update Subject
Request - Set new subject number
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/update' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States",
"site": "101",
"subject": "101-002",
"subject_new": "101-005"
}
]
}'
Response - Set new subject number
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"id": "OPP000000000501",
"study_country": "United States",
"site": "101",
"subject": "101-002",
"subject_new": "101-005",
"ixrs_id": "234-23_ABC",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000501&tab_name=data_entry__v"
}
]
}
Request - Update an IXRS id
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/update' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"study_country": "United States",
"site": "101",
"subject": "101-002",
"ixrs_id": "234-23_ABC_v2"
}
]
}'
Response - Update an IXRS id
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"id": "OPP000000000501",
"study_country": "United States",
"site": "101",
"subject": "101-002",
"subject_new": null,
"ixrs_id": "234-23_ABC_v2",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000501&tab_name=data_entry__v"
}
]
}
Notes
- Used to update the Subject number and/or IXRS id value.
- This endpoint uses the Study context (Study Country / Site / Subject) for identification.
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/update
Required Permissions
The following permissions are required to use the Update Subject API:
- API Access
- View Casebook
- Create Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
subjects |
Name of the Study Country of the Subject |
site |
subjects |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
subjects |
Name of the Subject (ID / number) now |
subject_new |
subjects |
Optional Name of the Subject (ID / number) to change to |
ixrs_id |
subjects |
Optional IXRS id value to update to |
Update Subject by ID
Request - Set new subject number
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/updatebyid' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"id": "OPP000000000501",
"subject_new": "101-005"
}
]
}'
Response - Set new subject number
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"id": "OPP000000000501",
"subject_new": "101-005",
"ixrs_id": "234-23_ABC",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000501&tab_name=data_entry__v"
}
]
}
Request - Update an IXRS id
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/updatebyid' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"subjects": [
{
"id": "OPP000000000501",
"ixrs_id": "234-23_ABC_v2"
}
]
}'
Response - Update an IXRS id
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "SUCCESS",
"id": "OPP000000000501",
"subject_new": null,
"ixrs_id": "234-23_ABC_v2",
"cdms_url": "/ui/#app/page/object-redirect?object_type=subject__v&object_id=114107_OPP000000000501&tab_name=data_entry__v"
}
]
}
Notes
- Used to update the Subject number and/or IXRS id value.
- This endpoint uses the internal Vault
idfor identification, which can be found using Retrieve Subjects
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects/actions/updatebyid
Required Permissions
The following permissions are required to use the Update Subject by ID API:
- API Access
- View Casebook
- Create Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
id |
subjects |
ID of the Subject |
subject_new |
subjects |
Optional Name of the Subject (ID / number) to change to |
ixrs_id |
subjects |
Optional IXRS id value to update to |
Event Groups
Clinical Data groups related Events - oftern referred to as visits - together in Event Groups. The group can contain one Event (e.g. unscheduled visit, screening visit), or many (e.g. treatment period). An Event Group can also repeat (e.g. cycles for a Study).
Create Event Groups
Request - Create Scheduled Event Group
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/eventgroups \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"eventgroups": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "main_visits"
}
]
}'
Response - Create Scheduled Event Group
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "main_visits"
}
]
}
Request - Create Unscheduled Event Group (requires a date)
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/eventgroups \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"eventgroups": [
{
"study_country": "United States"
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"date": "2022-03-03"
}
]
}'
Response - Create Unscheduled Event Group (requires a date)
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"date": "2022-03-03",
"externally_owned_date": true
}
]
}
Notes
- Used to add an Event Group for a Subject in the Study.
- This action will add the 'next' group, in sequence if the Event Group repeats. Example - for a repeating Event Group with two existing, the action will add another with
sequence= 3. - Use the Upsert Event Groups (PUT, next section), for conditional add at a specific sequence. Example - for a repeating Event Group with three existing, the attempt to add
sequence= 3 will take no action. - For Event Group types NOT Unscheduled, the date is not set in this API call. Instead, Set Event Date or Update Event are used.
- The limit for actions in one request is 100.
| Use Case | Description |
|---|---|
| Integration Only | Add of an area for integration data, that only the integration should control (not an end user). |
| Unscheduled | Unscheduled Event, which requires a date (format: yyyy-MM-dd) for the action. externally_owned_date = true will be set as a property on the date. |
| Cycles | These repeating Event Groups contain the same collection of Events and Forms for each sequence (repetition) of the Event Group, e.g. Day 1, Day 8, Day 15 visits (each cycle). Each new Event Group may have a customized label, such as Cycle 1, Cycle 2, Cycle 3, and so on. |
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/eventgroups
Required Permissions
The following permissions are required to use the Create Event Groups API:
API Access View Casebook Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
eventgroups |
Name of the Study Country of the Subject |
site |
eventgroups |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
eventgroups |
Name of the Subject (ID / number) where the Event Group is added |
eventgroup_name |
eventgroups |
Study design name of the Event Group |
date |
eventgroups |
Optional Date for the Event of the Event Group. Use "yyyy-mm-dd" format. This is only applicable to unscheduled type Event Groups. If omitted and an unscheduled Event Group, Vault sets the date to the current date. |
Upsert Event Groups
Request - Attempt when Event does not exist
curl -X PUT https://my-vault.veevavault.com/api/v24.1/app/cdm/eventgroups \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"eventgroups": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 1,
"date": "2022-03-03"
}
]
}'
Response - Attempt when Event does not exist
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 1,
"date": "2022-03-03",
"externally_owned_date": true
}
]
}
Request - Attempt to create sequence 2 and 3, with 2 already existing
curl -X PUT https://my-vault.veevavault.com/api/v24.1/app/cdm/eventgroups \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"eventgroups": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 2,
"date": "2022-04-03"
},
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 3,
"date": "2022-05-03"
}
]
}'
Response - Attempt to create sequence 2 and 3, with 2 already existing
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 2,
"date": "2022-04-03",
"externally_owned_date": true
},
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "unscheduled_treatment_visits",
"eventgroup_sequence": 3,
"date": "2022-05-03",
"externally_owned_date": true
}
]
}
Notes
- Used to upset an Event Group for a Subject in the Study.
- This action will add the 'next' group, in sequence if the Event Group does not yet exist, otherwise will skip the action. Example - for a repeating Event Group with two existing, the action will add another with
sequence= 3. - For cases where the Event Group already exists for the given
sequence, the response will indicateSUCCESS:UPDATEDdespite no action having taken place. - The Create Event Groups (POST, previous section) will return an error when used where the Event Group already exists for that Subject at that
sequence. - For Event Group types NOT Unscheduled, the date is not set in this API call. Instead, Set Event Date or Update Event are used.
- The limit for actions in one request is 100.
| Use Case | Description |
|---|---|
| Integration Only | Add of an area for integration data, that only the integration should control (not an end user). |
| Unscheduled | Unscheduled Event, which requires a date (format: yyyy-MM-dd) for the action. externally_owned_date = true will be set as a property on the date. |
| Cycles | Studies with Cycles - these repeating Event Groups contain the same collection of Events and Forms for each sequence (repetition) of the Event Group, e.g. Day 1, Day 8, Day 15 visits (each cycle). Each new Event Group may have a customized label, such as Cycle 1, Cycle 2, Cycle 3, and so on. |
Endpoint
PUT /api/{version}/app/cdm/eventgroups
Required Permissions
The following permissions are required to use the Upsert Event Groups API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
eventgroups |
Name of the Study Country of the Subject |
site |
eventgroups |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
eventgroups |
Name of the Subject (ID / number) where the Event Group is added |
eventgroup_name |
eventgroups |
Study design name of the Event Group |
eventgroup_sequence |
eventgroups |
Sequence of the Event Group that should be added (if does not exist), otherwise skipped |
date |
eventgroups |
Optional Date for the Event Group. Use "yyyy-mm-dd" format. This is only applicable to unscheduled type Event Groups. If omitted and an unscheduled Event Group, Vault sets the date to the current date. |
Events
Clinical Data tracks visits as an Event. Each Event contains the Forms collected during that visit. The parent container of an Event is an Event Group. When an Event repeats, it is because the Event Group it resides within repeats. The sequence number in the hierarchy for a repeating Event is at the Event Group level.
Retrieve Events / Forms
Request
curl -L -X GET 'https://myvault.veevavault.com/api/v24.1/app/cdm/events?study_name=ABCP-2022-01_DEV1&study_country=United%20States&site=0101&subject=0101-0003' \
-H 'Authorization: [SESSION_ID]' \
-H 'Content-Type: application/json'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 5,
"total": 5
},
"events": [
{
"id": "OPS00000001M006",
"study_country": "United States",
"site": "0101",
"subject": "0101-0003",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1,
"locked": false,
"frozen": false,
"event_date": "2020-02-03",
"externally_owned_date": true,
"event_did_not_occur": false,
"method": "on_site_visit__v",
"externally_owned_method": false,
"forms": [
{
"id": "OPT00000001M002",
"form_name": "demographics",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-07-11T17:38:15Z",
"last_submit_date": "2023-07-11T17:38:15Z",
"intentionally_left_blank": false
},
{
"id": "OPT00000001M003",
"form_name": "exclusion_criteria",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-07-11T17:38:15Z",
"last_submit_date": "2023-07-14T12:11:33Z",
"intentionally_left_blank": false
},
{
"id": "OPT00000001M004",
"form_name": "inclusion_criteria",
"form_sequence": 1,
"form_status": "submitted__v",
"first_submit_date": "2023-08-25T15:22:33Z",
"last_submit_date": "2023-08-25T15:22:33Z",
"locked": false,
"frozen": false,
"intentionally_left_blank": true,
"intentionally_left_blank_reason": "Information not available"
}
]
},
{
"id": "OPS00000001M009",
"study_country": "United States",
"site": "0101",
"subject": "0101-0003",
"eventgroup_name": "re_screen",
"eventgroup_sequence": 1,
"event_name": "baseline_visit",
"event_sequence": 1,
"locked": false,
"frozen": false,
"event_date": "",
"externally_owned_date": false,
"event_did_not_occur": true,
"event_did_not_occur_reason": "Subject passed screening initially",
"method": null,
"externally_owned_method": false,
"forms": []
},
{
"id": "OPS00000001M011",
"study_country": "United States",
"site": "0101",
"subject": "0101-0003",
"eventgroup_name": "enrollment",
"eventgroup_sequence": 1,
"event_name": "enrollment_visit",
"event_sequence": 1,
"locked": false,
"frozen": false,
"event_date": "2020-02-07",
"externally_owned_date": false,
"event_did_not_occur": false,
"method": "on_site_visit__v",
"externally_owned_method": true,
"forms": [
{
"id": "OPT00000001M007",
"form_name": "randomization",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-08-29T15:22:33Z",
"last_submit_date": "2023-08-29T15:22:33Z",
"intentionally_left_blank": false
}
]
},
{
"id": "OPS00000001M008",
"study_country": "United States",
"site": "0101",
"subject": "0101-0003",
"eventgroup_name": "treatment_visit",
"eventgroup_sequence": 1,
"event_name": "treatment_visit",
"event_sequence": 1,
"locked": false,
"frozen": false,
"event_date": "",
"externally_owned_date": false,
"event_did_not_occur": false,
"method": null,
"externally_owned_method": false,
"forms": []
},
{
"id": "OPS00000001M007",
"study_country": "United States",
"site": "0101",
"subject": "0101-0003",
"eventgroup_name": "common_forms",
"eventgroup_sequence": 1,
"event_name": "common_forms",
"event_sequence": 1,
"locked": false,
"frozen": false,
"event_date": "",
"externally_owned_date": false,
"event_did_not_occur": false,
"method": null,
"externally_owned_method": false,
"forms": []
}
]
}
Notes
- Retrieve a list of Events, and the Forms within, for a specific Subject. At current release, request is limited to one Subject. Filtering is available down to the Event level. For retrieval of more than one Subject (or many Subjects), see the various export options at the Job Summary / Types section.
- Vault only returns details about Forms that you have permission to view. If the Event contains restricted (blinded) Forms, and you do not have the Restricted Data Access permission, Vault will not return the restricted Form.
- Event method is a property of Events that depends on the Study design, both whether the Event uses methods and which values are valid at that Event.
- The return array
formswithin each Event can be empty for several reasons:- The Event was specifically set as Did Not Occur by a site user, with reason
- The Event is still in planned state, i.e., no Event date is yet set.
- The Event is of type log, and the Forms within it are all part of Study rules, none of which have evaluated as true for the Subject yet.
Endpoint
GET /api/{version}/app/cdm/events
Required Permissions
The following permissions are required to use the Retrieve Events API:
- API Access
- View Casebook
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
study_country |
Name of the Study Country for the site of the Subject |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
Name (ID/number) of the Subject |
eventgroup_name |
Optional Study design name for a specific Event Group to filter the return on |
eventgroup_sequence |
Optional Specific sequence to filter the return on |
event_name |
Optional Study design name for a specific Event to filter the return on |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Events and Forms within for a single Subject. Details of the response:
| Array | Name | Description |
|---|---|---|
events |
id |
Vault internal ID for the Event (will never change) |
events |
study_country |
Name of the Study Country for the site of the Subject |
events |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
events |
subject |
Name (ID/number) of the Subject |
events |
eventgroup_name |
Study design name for the Event Group |
events |
eventgroup_sequence |
Sequence of the Event Group of the Event. If its a repeating Event Group, the value increments for each instance, starting at "1", then "2", and so on. If the Event Group isn't repeating, Vault returns "1". |
events |
event_name |
Study design name for a the Event |
events |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level |
events |
event_date |
Event date of the Event. yyyy-MM-dd format is returned. |
events |
externally_owned_date |
Whether the date is owned by an external system, i.e. read only to the site users. |
events |
event_did_not_occur |
Whether the event has been marked as not having occurred by a site user. (true/false) |
events |
event_did_not_occur_reason |
Only when event_did_not_occur is true, otherwise this is omitted. |
events |
method |
The method of the event/visit, e.g. on_site_visit__v. Methods are configured at specific Events and with specific choices at those Events. Consult the Study Design for when/if they apply at any of the Events for the Study |
events |
externally_owned_method |
Whether the visit method is owned by an external system, i.e. read only to the site users. |
events/forms |
id |
Vault internal ID for the Form (will never change) |
events/forms |
form_name |
Study design name for a Form in the Event (entry per form) |
events/forms |
form_sequence |
If its a repeating Form, the value increments for each instance, starting at "1", then "2", and so on. If the Form isn't repeating, Vault returns "1". |
events/forms |
form_status |
The status (picklist name) of that form (e.g. blank__v, submitted__v, etc. See the table below for possible form statuses. |
events/forms |
locked |
Whether the form is locked (true/false). Locked Forms cannot be updated, nor the queries on them |
events/forms |
frozen |
Whether the form is locked (true/false). Frozen Forms cannot be updated (data wise), queries can be updated / added. |
events/forms |
first_submit_date |
The first time the form was submitted. |
events/forms |
last_submit_date |
The most recent time the form was submitted. |
events/forms |
intentionally_left_blank |
Whether the Form has been marked intentionally left blank (ILB) by a site user. (true/false) |
events/forms |
intentionally_left_blank_reason |
When intentionally_left_blank is true for the Form, the reason. Otherwise, omitted. |
Form Statuses
| Status | API Value | Notes |
|---|---|---|
| Blank | blank__v |
The form is not yet visited (no data points / items), or is visited but all fields are their initial blank status. The form can also take on this status after a reset action |
| In Progress | in_progress__v |
At least one data point (item) on the form has its first value. |
| Submitted | submitted__v |
The form is in submitted/completed state. No updates (UI or API) can occur until the form is opened for edit. NOTE: A form can be in submitted status and be additionally marked Intentionally Left Blank. (i.e., reasons for not having the data) |
| In Progress Post Submit | in_progress_post_submit__v |
Like in_progress__v, but the form has been submitted (then reopened) at least once. |
Create Events
Request - Create two events, different subjects
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.RANDOMIZATION",
"event_name": "Subject.Information"
},
{
"study_country": "United States",
"site": "101",
"subject": "101-002",
"eventgroup_name": "EG.SCREENING",
"event_name": "Demographics"
}
]
}
Response - Create two events, different subjects
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.RANDOMIZATION",
"eventgroup_sequence": 1,
"event_name": "Subject.Enrollment",
"event_sequence": 1
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-002",
"eventgroup_name": "EG.SCREENING",
"eventgroup_sequence": 1,
"event_name": "Subject.ScreeningVisit",
"event_sequence": 1
}
]
}
Request - Create event inside a repeating event group
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.CYCLE_EVENTS",
"eventgroup_sequence": 3,
"event_name": "evDAY15"
}
]
}
Response - Create event inside a repeating event group
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.CYCLE_EVENTS",
"eventgroup_sequence": 3,
"event_name": "evDAY15"
"event_sequence": 1
}
]
}
Notes
- Used to create an Event inside an existing Event Group,typically for a dynamic or ‘integration only’) reasons.
- The action does not add a non-existent Event Group where the Event otherwise is to reside. See Create Event Groups / Upsert Event Groups for the addition on a non-existent Event Group prior to creating the Event.
- The action does not set the Event date or visit method. Instead, Set Event Date or Update Event are used.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/events
Required Permissions
The following permissions are required to use the Create Events API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
events |
Name of the Study Country of the Subject |
site |
events |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
events |
Name of the Subject (ID / number) where the Event is added |
eventgroup_name |
events |
Study design name of the Event Group |
eventgroup_sequence |
events |
Optional If omitted, 1 is assumed. Use this parameter to specify an Event of a repeating Event Group |
event_name |
events |
Study design name of the Event to add |
Set Event Date
Request - Set one event date
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/setdate \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"event_name": "Screening-Visit",
"date": "2022-05-21"
}
]
}'
Response - Set one event date
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"id": "OPS00000001M006",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"eventgroup_sequence": 1,
"event_name": "Screening-Visit",
"event_sequence": 1,
"date": "2022-05-21",
"externally_owned_date": true,
"allow_planneddate_override": false,
"change_reason": "Action performed via the API"
}
]
}
Request - Change one event date
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/setdate \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"event_name": "Screening-Visit",
"date": "2022-05-22",
"change_reason": "Updated by integration - my reason"
}
]
}'
Response - Change one event date
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"id": "OPS00000001M006",
"study_country": "United States",
"site": "101",
"subject": "SCR-0002",
"eventgroup_name": "Screening-Visit",
"eventgroup_sequence": 1,
"event_name": "Screening-Visit",
"event_sequence": 1,
"date": "2022-05-22",
"externally_owned_date": true,
"allow_planneddate_override": false,
"change_reason": "Updated by integration - my reason"
}
]
}
Notes
- Used to set an Event date to an existing Event.
- Clinical Data use an Event date above all Forms within it, i.e., the date applies to each Form. This is as opposed to having visit/event dates inside each Form of an Event. The Event date will be automatically available in data exports.
- The action does not add the Event or Event Group the Event otherwise resides
- Date format is yyyy-MM-dd
- The endpoint call (as POST) is used for both setting a date when no value currently exists, plus updating an existing value. (PUT is not used for update)
- For an Unscheduled type Event Group, the date of the first Event of the group is set at the time of create / upsert of the Event Group.
- For updating of an Event method see the next section - Update Event - where both the date and method can be updated.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/events/actions/setdate
Required Permissions
The following permissions are required to use the Set Event Date API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
events |
Name of the Study Country of the Subject |
site |
events |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
events |
Name of the Subject (ID / number) |
eventgroup_name |
events |
Study design name of the Event Group |
eventgroup_sequence |
events |
Optional If omitted, 1 is assumed. Use this parameter to specify an Event of a repeating Event Group |
event_name |
events |
Study design name of the Event to add. |
date |
events |
The Event date to set, using yyyy-MM-dd format |
change_reason |
events |
Optional The reason for change of an Event's date, where there is already a value. For example, "Date updated by remote system". When not provided and a reason is required, the system will use a default reason of Action performed via the API |
allow_planneddate_override |
events |
Optional Set this to true to bypass checking on the Event planned schedule (window/range). Otherwise, an attempt with date outside the planned range will return an error. When omitted, false is assumed. |
externally_owned_date |
events |
Optional When omitted true is used, i.e. the site user cannot then further edit the date. Pass a false to re-enable the ability for a user to edit a previously read only Event date. |
Update Event
Request - Update one event date (no method)
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/update \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"event_name": "Screening-Visit",
"date": "2023-12-21"
}
]
}'
Response - Update one event date (no method)
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"id": "OPS00000001M006",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"eventgroup_sequence": 1,
"event_name": "Screening-Visit",
"event_sequence": 1,
"date": "2023-12-21",
"method": null,
"allow_planneddate_override": false,
"externally_owned_date": true,
"externally_owned_method": false
"change_reason": "Action performed via the API"
}
]
}
Request - Update date and method
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/update \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Screening-Visit",
"event_name": "Screening-Visit",
"date": "2023-12-22",
"method": "on_site_visit__v",
"change_reason": "Updated by integration - my reason"
}
]
}'
Response - Update date and method
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"id": "OPS00000001M006",
"study_country": "United States",
"site": "101",
"subject": "SCR-0002",
"eventgroup_name": "Screening-Visit",
"eventgroup_sequence": 1,
"event_name": "Screening-Visit",
"event_sequence": 1,
"date": "2023-23-22",
"method": "on_site_visit__v",
"allow_planneddate_override": false,
"externally_owned_date": true,
"externally_owned_method": true,
"change_reason": "Updated by integration - my reason"
}
]
}
Notes
- Used to set an Event date and, where applicable, a visit method for an existing Event. Note: The study design determines which Events accept a method and which methods are valid for each Event. Consult your study design to understand when a method is required. The method must be provided where configured. If a method is required but not provided, the Set Event Date endpoint will fail for those Events.
- Clinical Data uses an Event date above all Forms within it, i.e., the date applies to each Form. This is as opposed to having visit/event dates inside each Form of an Event. The Event date will be automatically available in data exports.
- Event method is a property of Events that depends on the Study design, both whether the Event uses methods and which values are valid at that Event.
- The action does not add the Event or Event Group the Event otherwise resides
- Date format is yyyy-MM-dd
- The endpoint call (as POST) is used for both setting a date when no value currently exists, plus updating an existing value. (PUT is not used for update)
- For method, updates from no value to a value or existing value to no value are both allowed. This is as opposed to the date, which most always be given, even if the intent is just to update a method. Special situations with
methodare shown in the table below. - For an Unscheduled type Event Group, the date of the first Event of the group is set at the time of create / upsert of the Event Group.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/events/actions/setdate
Required Permissions
The following permissions are required to use the Set Event Date API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
events |
Name of the Study Country of the Subject |
site |
events |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
events |
Name of the Subject (ID / number) |
eventgroup_name |
events |
Study design name of the Event Group |
eventgroup_sequence |
events |
Optional If omitted, 1 is assumed. Use this parameter to specify an Event of a repeating Event Group |
event_name |
events |
Study design name of the Event to add. |
date |
events |
The Event date to set, using yyyy-MM-dd format |
method |
events |
The method to set, or use *null to unset a previously set value. The value used is dependent on the Study Design, both if allowed at the Event and which values are valid. |
change_reason |
events |
Optional The reason for change of an Event's date, where there is already a value. For example, "Date updated by remote system". When not provided and a reason is required, the system will use a default reason of Action performed via the API |
allow_planneddate_override |
events |
Optional Set this to true to bypass checking on the Event planned schedule (window/range). Otherwise, an attempt with date outside the planned range will return an error. When omitted, false is assumed. |
externally_owned_date |
events |
Optional When omitted true is used, i.e. the site user cannot then further edit the date. Pass a false to re-enable the ability for a user to edit a previously read only Event date. |
externally_owned_method |
events |
Optional When omitted true is used if the update includes setting of a method. The site user cannot then further edit the value. Pass a false to re-enable the ability for a user to edit a previously read only method. |
Set Event as Did Not Occur
Request - Set two events as did not occur
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/didnotoccur \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"events": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.MAIN",
"eventgroup_sequence": 1,
"event_name": "EV.V7",
"change_reason": "missed visit"
},
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.MAIN",
"eventgroup_sequence": 1,
"event_name": "EV.V8",
"change_reason": "missed visit"
}
]
}
Response - Set two events as did not occur
{
"responseStatus": "SUCCESS",
"events": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.MAIN",
"eventgroup_sequence": 1,
"event_name": "EV.V7",
"event_sequence": 1
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.MAIN",
"eventgroup_sequence": 1,
"event_name": "EV.V8",
"event_sequence": 1
}
]
}
Notes
- Used to mark an entire Event with status = Did Not Occur (
did_not_occur__v) - Change of reason can be any text, although certainly consider being consistent verbiage on API requests.
- Example: Subject missed the visit, Subject discontinued from the study, etc.
- A reason for this action is required.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/events/actions/didnotoccur
Required Permissions
The following permissions are required to use the Set Event as Did Not Occur API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
events |
Name of the Study Country of the Subject |
site |
events |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
events |
Name of the Subject (ID / number) |
eventgroup_name |
events |
Study design name of the Event Group |
eventgroup_sequence |
events |
Optional If omitted, 1 is assumed. Use this parameter to specify an Event of a repeating Event Group |
event_name |
events |
Study design name of the Event to add. |
change_reason |
events |
Optional The specific reason to set when marking the Event as Did Not Occur. For example: "Update by integration, subject missed the event." |
Forms
Clinical Data uses Forms to hold like data. Inside the Form are Item Groups (grouping of fields/controls) and Items (fields for a single data point). An Item Group can repeat inside a single form, as per study design needs. Learn more about data entry into Forms in Clinical Data Help.
Retrieve Forms / Item Data
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/forms?study_name=ABCP-2022-01_DEV1&site=0101&study_country=United%20States&subject=101-001&eventgroup_name=screening&event_name=screening_visit' \
-H 'Authorization:{SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 4,
"total": 4
},
"forms": [
{
"id": "OPT00000001M002",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1,
"event_external_id": "screening_visit",
"form_name": "demographics",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-07-11T17:38:15Z",
"last_submit_date": "2023-07-14T12:11:33Z",
"intentionally_left_blank": false,
"itemgroups": [
{
"id": "V5C00000000B007",
"itemgroup_name": "creation_criteria",
"itemgroup_sequence": 1,
"itemgroup_external_id": "creation_criteria",
"items": [
{
"id": "V5D00000000B031",
"item_name": "subject_date_of_birth",
"value": "17-Jun-1978",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B032",
"item_name": "subject_gender",
"value": "F",
"intentionally_left_blank": false,
"externally_owned": true,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B033",
"item_name": "subject_initials",
"value": "QGT",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B034",
"item_name": "subject_race",
"value": "Caucasian",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B035",
"item_name": "weight",
"value": "150.2",
"unit_value": "lbs",
"translated_value": "68.129574",
"translated_unit_value": "kg",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
}
]
}
]
},
{
"id": "OPT00000001M003",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1,
"event_externalid": "screening_visit",
"form_name": "exclusion_criteria",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-07-28T12:11:33Z",
"last_submit_date": "2023-07-28T12:11:33Z",
"intentionally_left_blank": false,
"itemgroups": [
{
"id": "V5C00000000B008",
"itemgroup_name": "exclusion_criteria",
"itemgroup_sequence": 1,
"itemgroup_externalid": "exclusion_criteria",
"items": [
{
"id": "V5D00000000B017",
"item_name": "exclusion_hypersensitivity",
"value": "N",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B018",
"item_name": "exclusion_study_calendar",
"value": "N",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B019",
"item_name": "exclusion_pregnant_or_nursing",
"value": "N",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B020",
"item_name": "exclusion_chronic_medical_condition",
"value": "N"
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
}
]
}
]
},
{
"id": "OPT00000001M004",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1,
"event_externalid": "screening_visit",
"form_name": "inclusion_criteria",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-08-02T17:38:15Z",
"last_submit_date": "2023-08-02T17:38:15Z",
"intentionally_left_blank": false,
"itemgroups": [
{
"id": "V5C00000000B009",
"itemgroup_name": "inclusion_criteria",
"itemgroup_sequence": 1,
"itemgroup_externalid": "inclusion_criteria",
"items": [
{
"id": "V5D00000000B021",
"item_name": "inclusion_negative_pregnancy_test",
"value": "Y",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B022",
"item_name": "inclusion_normal_healthy",
"value": "Y",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B023",
"item_name": "inclusion_age_18",
"value": "Y",
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B024",
"item_name": "inclusion_informed_consent",
"value": "Y".
"intentionally_left_blank": false,
"externally_owned": false,
"frozen": false,
"locked": false
}
]
}
]
},
{
"id": "OPT00000001M005",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1,
"event_externalid": "screening_visit",
"form_name": "inclusion_criteria",
"form_sequence": 1,
"form_status": "submitted__v",
"locked": false,
"frozen": false,
"first_submit_date": "2023-07-11T19:38:15Z",
"last_submit_date": "2023-07-11T19:38:33Z",
"intentionally_left_blank": true,
"intentionally_left_blank_reason": "Did not complete",
"itemgroups": [
{
"id": "V5C00000000B010",
"itemgroup_name": "pk_1",
"itemgroup_sequence": 1,
"itemgroup_externalid": "pk_1",
"items": [
{
"id": "V5D00000000B045",
"item_name": "pk_date",
"value": null,
"intentionally_left_blank": true,
"intentionally_left_blank_reason": "Did not complete",
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B046",
"item_name": "pk_result",
"value": null,
"intentionally_left_blank": true,
"intentionally_left_blank_reason": "Did not complete",
"externally_owned": false,
"frozen": false,
"locked": false
},
{
"id": "V5D00000000B047",
"item_name": "pk_not_done_reason",
"value": null,
"intentionally_left_blank": true,
"intentionally_left_blank_reason": "Did not complete",
"externally_owned": false,
"frozen": false,
"locked": false
}
]
}
]
}
]
}
Notes
- Use this API to retrieve existing Forms and their data (Items, Item Groups). Details of the Form status, frozen/locked/ILB are also returned.
- Typically used for specific inspection of a Form (with data on it), prior to updating the Form using other endpoints.
- Vault only returns details about Forms that you have permission to view. If the Event contains restricted (blinded) Forms, and you do not have the Restricted Data Access permission, Vault will not return the restricted Form.
- The largest scope of the retrieval is one Subject, and one Event of the Subject
- For retrieval of more than one Event or many Subjects, see the various export options at the Job Summary / Types section.
- The
externally_ownedproperty for each item will returntruewhen the Item was set via the API. This prevents updates of the field by the Site users.
Endpoint
GET /api/{version}/app/cdm/forms
Required Permissions
The following permissions are required to use the Retrieve Forms API:
- API Access
- View Casebook
The CDMS API Read Only and CDMS API Read Write roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
study_country |
Name of the Study Country for the site of the Subject |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
Name (ID/number) of the Subject |
eventgroup_name |
Study design name for an Event Group |
eventgroup_sequence |
Optional Specific sequence to filter the Event Group on. If omitted, 1 is assumed. |
event_name |
Study design name for an Event (of that Event Group) |
form_name |
Optional Study design name of a Form (in the Event) to filter on |
form_sequence |
Optional Specific sequence to filter on, that is, form of a specific sequence. If omitted, 1 is assumed. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Forms within the Subject / Event provided, with the following information:
| Array | Name | Description |
|---|---|---|
forms |
id |
Vault internal ID for the Form (will never change) |
forms |
study_country |
Name of the Study Country for the site of the Subject |
forms |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
forms |
subject |
Name (ID/number) of the Subject |
forms |
eventgroup_name |
Study design name for the Event Group |
forms |
eventgroup_sequence |
Sequence of the Event Group of the Event. If repeating Event Group the value increments starting at 1, 2.... If it does not repeat, then 1 is always returned |
forms |
event_name |
Study design name for a the Event |
forms |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level |
forms |
event_date |
Event date of the Event. yyyy-MM-dd format is returned. |
forms |
form_name |
Study design name for a Form in the Event (entry per form) |
forms |
form_sequence |
If the Form repeats per study design, these increment starting at 1, 2.... If it does repeat, 1 is always returned. |
forms |
form_status |
The status (picklist name) of that form (e.g. blank__v, submitted__v, etc. See the table below for possible form statuses. |
forms |
locked |
Whether the form is locked (true/false). Locked Forms cannot be updated, nor the queries on them |
forms |
frozen |
Whether the form is locked (true/false). Frozen Forms cannot be updated (data wise), queries can be updated / added. |
forms |
first_submit_date |
The first time the form was submitted. |
forms |
last_submit_date |
The most recent time the form was submitted. |
forms |
intentionally_left_blank |
Whether the Form has been marked intentionally left blank (ILB) by a site user. (true/false) |
forms |
intentionally_left_blank_reason |
When intentionally_left_blank is true for the Form, the reason. Otherwise, omitted. |
forms/itemgroups |
id |
Vault internal ID for the Item Group (will never change) |
forms/itemgroups |
itemgroup_name |
Study design name for an Item Group in the Form |
forms/itemgroups |
itemgroup_sequence |
If the Item Group repeats per study design (within the Form), these increment starting at 1, 2.... If it does repeat, 1 is always returned. |
forms/itemgroups |
itemgroup_external_id |
Another study design reference/name for the Item Group. This value is usually the same as the name. |
forms/itemgroups/items |
id |
Vault internal ID for the Item (will never change) |
forms/itemgroups/items |
item_name |
Study design name for the Item |
forms/itemgroups/items |
value |
The value for that Item. The value returned will differ by the type of the Item, study design wise (e.g. Date, DateTime, Time, Check Box, Codelist, etc., see the table below) |
forms/itemgroups/items |
unit_value |
Only applies when the Item type is Unit Codelist. This is the unit selected to pair with the value entered by the end user. If the Item is not a Unit Codelist, this line is omitted. |
forms/itemgroups/items |
translated_value |
Only applies when the Item type is Unit Codelist. The user value is calculated to the standard unit defined for the Item. If the user selects the standard unit, the value here will be the same as value. If the Item is not a Unit Codelist, this line is omitted. |
forms/itemgroups/items |
translated_unit_value |
Only applies when the Item type is Unit Codelist. The standard unit for the Item, as defined in the study. If the user selects the standard unit, the value here will be the same as unit_value. If the Item is not a Unit Codelist, this line is omitted. |
forms/itemgroups/items |
lab_modifier |
Only applies when the Item type is Unit Codelist, and the Item has a value for Lab Modifier. Example: A lab value is 'Less than 0.7', results in value: 1, and the coded value for 'Less than' as setup in the study design. If the Item is not a Unit Codelist or the Lab Modifier is empty / not set, this line is omitted. |
forms/itemgroups/items |
intentionally_left_blank |
Whether the Item has been marked intentionally left blank (ILB) by a site user. (true/false) |
forms/itemgroups/items |
intentionally_left_blank_reason |
When intentionally_left_blank is true for the Item, the reason. Otherwise, omitted. |
forms/itemgroups/items |
externally_owned |
Whether the Item is owned by another system. When true, the Item value it not editable by site users, even if the Item Definition is set for editable. |
forms/itemgroups/items |
frozen |
Whether the Item is currently frozen or not. An Item can be frozen without freezing the entire Form. Frozen does not allow data changes, but does allow Query activity. |
forms/itemgroups/items |
locked |
Whether the Item is currently locked or not. An Item can be locked without freezing the entire Form. Locked does not allow data changes, nor Query activity. |
Form Statuses
| Status | API Value | Notes |
|---|---|---|
| Blank | blank__v |
The form is not yet visited (no data points / items), or is visited but all fields are their initial blank status. The form can also take on this status after a reset action |
| In Progress | in_progress__v |
At least one data point (item) on the form has its first value. |
| Submitted | submitted__v |
The form is in submitted/completed state. No updates (UI or API) can occur until the form is opened for edit. NOTE: A form can be in submitted status and be additionally marked Intentionally Left Blank. (i.e., reasons for not having the data) |
| In Progress Post Submit | in_progress_post_submit__v |
Like in_progress__v, but the form has been submitted (then reopened) at least once. |
Item Value Return by Item Type
| Item Type | State/Value | Example Return |
|---|---|---|
| Checkbox | (never been checked) | "value": null |
| Checkbox | Checked | "value": "true" |
| Checkbox | Unchecked (but at one time checked) | "value": "false" |
| Codelist | (never been selected) | "value": null |
| Codelist | Codelist code value | "value": "Y" (e.g. 'Yes' = 'Y' for codelist) |
| Codelist | Codelist saved non-empty, now empty | "value": null |
| Date | (never been set) | "value": null |
| Date | Full date set | "value": "05-Jun-2022" (The study date format is returned, not ISO) |
| Date | (value set, then later unset) | "value": null |
| Date | Partial date saved (day and month unknown) | "value": "UN-UNK-2022" (assuming dd-MMM-yyyy study date format) |
| DateTime | (never been set) | "value": null |
| DateTime | Full date time set | "value": "05-Jun-2022 13:30" (The study date format is returned, not ISO) |
| DateTime | (value set, then later unset) | "value": null |
| DateTime | Partial date saved (day and time unknown) | "value": "UN-Jun-2022 UN:UN" Assuming dd-MMM-yyyy study date format, day and time unknown |
| URL Field | (never been set) | "value": null |
| URL Field | Set with a value | "value": "https://www.google.com" |
| URL Field | (value set, then later unset) | "value": null |
| Unit Codelist | (never been set) | "value": null, "unit_value": null, "translated_value": null, "translated_unit_value": null |
| Unit Codelist | Set with value and unit | "value": "150.2", "unit_value": "lbs", "translated_value": "68.129574", "translated_unit_value": "kg" |
| Unit Codelist | Set with value and unit, plus lab modifier set | "value": "0.001" "unit_value": "mg", "translated_value": "1.0", "translated_unit_value": "g", "lab_modifier": "<" |
| Unit Codelist | (value set, then later unset) | "value": null, "unit_value": null, "translated_value": null, "translated_unit_value": null |
| Numeric (Integer or Decimal) | (never been set) | "value": null |
| Numeric (Integer or Decimal) | Set with a value | "value": "32.45" |
| Numeric (Integer or Decimal) | (value set, then later unset) | "value": null |
| Text | (never been set) | "value": null |
| Text | Set with a value | "value": "This is my text set in the field on screen" |
| Text | Set with a value - comments field with return character (use \n for breaks) | "value": "Here is my long text with a carriage return \nhere \nand here\nand the end of the text is here" |
| Text | (value set, then later unset) | "value": null |
Combination Update Form Data
Request - Set data with a reopen and submit at the end
curl -L -X POST 'https://my-vault.veevavault.com/api{version}cdm/forms/actions/setdata' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"change_reason": "Integration Update",
"form": {
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"form_name": "DM",
"form_sequence": 1,
"itemgroups": [
{
"itemgroup_name": "igBASE_DM",
"itemgroup_sequence": 1,
"items": [
{
"item_name": "BIRTHYR",
"value": "1976"
},
{
"item_name": "SEX",
"value": "M"
}
]
}
]
}
}'
Response - Set data with a reopen and submit at the end
{
"responseStatus": "SUCCESS",
"reopen": true,
"submit": true,
"change_reason": "Integration Update",
"externally_owned": true,
"form": {
"id": "OPT000000000507",
"form_status": "submitted__v",
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "DM",
"form_sequence": 1,
"itemgroups": [
{
"responseStatus": "SUCCESS",
"id": "V54000000006002",
"itemgroup_name": "igBASE_DM",
"itemgroup_sequence": 1,
"items": [
{
"responseStatus": "SUCCESS",
"id": "V55000000006005",
"item_name": "BIRTHYR",
"value": "1976"
},
{
"responseStatus": "SUCCESS",
"id": "V55000000006006",
"item_name": "SEX",
"value": "M"
}
]
}
]
}
}
Request - lacking sequence defaults, change reason (defaults used)
curl -L -X POST 'https://my-vault.veevavault.com/api{version}cdm/forms/actions/setdata' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"form": {
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "DM",
"itemgroups": [
{
"itemgroup_name": "igBASE_DM",
"items": [
{
"item_name": "BIRTHYR",
"value": "1976"
},
{
"item_name": "SEX",
"value": "M"
}
]
}
]
}
}
Response - lacking sequence defaults, change reason (defaults used)
{
"responseStatus": "SUCCESS",
"reopen": true,
"submit": true,
"change_reason": "Action performed via the API",
"externally_owned": true,
"form": {
"id": "OPT000000000507",
"form_status": "submitted__v",
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "DM",
"form_sequence": 1,
"itemgroups": [
{
"responseStatus": "SUCCESS",
"id": "V54000000006002",
"itemgroup_name": "igBASE_DM",
"itemgroup_sequence": 1,
"items": [
{
"responseStatus": "SUCCESS",
"id": "V55000000006005",
"item_name": "BIRTHYR",
"value": "1976"
},
{
"responseStatus": "SUCCESS",
"id": "V55000000006006",
"item_name": "SEX",
"value": "M"
}
]
}
]
}
}
Request - Setting values for one of each type of item
curl -L -X POST 'https://my-vault.veevavault.com/api{version}cdm/forms/actions/setdata' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"form": {
"study_country": "United States",
"site": "101",
"subject": "101-101",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"form_name": "ONE_EACH_FORM",
"form_sequence": 1,
"itemgroups": [
{
"itemgroup_name": "igONE_EACH_TYPE",
"itemgroup_sequence": 1,
"items": [
{
"item_name": "CB",
"value": "true"
},
{
"item_name": "CL",
"value": "Y"
},
{
"item_name": "DATEONLY",
"value": "2022-06-01"
},
{
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN"
},
{
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z"
},
{
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ"
},
{
"item_name": "INTFIELD",
"value": "3"
},
{
"item_name": "DECIMAL",
"value": "5.2"
},
{
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std"
},
{
"item_name": "URL_FIELD",
"value": "https://www.google.com"
},
{
"item_name": "SHORTTXT",
"value": "Some text short"
},
{
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload"
}
{
"item_name": "LINK_TO_OTHER_FORM",
"value": "",
"item_to_form_link": {
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "MH",
"form_sequence": 3
}
}
]
}
]
}
}'
Response - Setting values for one of each type of item
{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"change_reason": "Action performed via the API",
"externally_owned": true,
"form": {
"id": "OPT000000000507",
"form_status": "submitted__v",
"study_country": "United States",
"site": "101",
"subject": "101-101",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"form_name": "ONE_EACH_FORM",
"form_sequence": 1,
"itemgroups": [
{
"responseStatus": "SUCCESS",
"id": "V54000000006002",
"itemgroup_name": "igONE_EACH_TYPE",
"itemgroup_sequence": 1,
"items": [
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "CB",
"value": "true"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "CL",
"value": "Y"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "DATEONLY",
"value": "2022-06-01"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "INTFIELD",
"value": "3"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "DECIMAL",
"value": "5.2"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "URL_FIELD",
"value": "https://www.google.com"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "SHORTTXT",
"value": "Some text short"
},
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload"
}
{
"responseStatus": "SUCCESS",
"id": "VB67000000006002",
"item_name": "LINK_TO_OTHER_FORM",
"value": "",
"item_to_form_link": {
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "MH",
"form_sequence": 3
}
}
]
}
]
}
}
Request - Failure at the item level, and bubble up to higher levels. The form was opened, and left open
curl -L -X POST 'https://my-vault.veevavault.com/api{version}cdm/forms/actions/setdata' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"form": {
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "DM",
"itemgroups": [
{
"itemgroup_name": "igBASE_DM",
"items": [
{
"item_name": "BIRTHYR",
"value": "1976"
},
{
"item_name": "SEX",
"value": "MM"
}
]
}
]
}
}
Response - Failure at the item level, and bubble up to higher levels. The form was opened, and left open
{
"responseStatus": "FAILURE",
"errorMessage": "One or more [Item] updates failed",
"reopen": true,
"submit": true,
"change_reason": "Action performed via the API",
"externally_owned": true,
"form": {
"id": "OPT000000000507",
"form_status": "in_progress_post_submit__v",
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "DM",
"form_sequence": 1,
"itemgroups": [
{
"responseStatus": "FAILURE",
"errorMessage": "One or more [Item] updates failed",
"itemgroup_name": "igBASE_DM",
"itemgroup_sequence": 1,
"items": [
{
"responseStatus": "SUCCESS",
"id": "V55000000006005",
"item_name": "BIRTHYR",
"value": "1976"
},
{
"responseStatus": "FAILURE",
"errorMessage": "[Codelist Item Definition] with name [MM] not found",
"item_name": "SEX",
"value": "MM"
}
]
}
]
}
}
Request - Form is frozen (no actions happen), top level reported
curl -L -X POST 'https://my-vault.veevavault.com/api{version}cdm/forms/actions/setdata' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_name": "ABC-XYZ-001_DEV1",
"reopen": true,
"submit": true,
"form": {
"study_country": "United States",
"site": "001",
"subject": "101-103",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "DM",
"itemgroups": [
{
"itemgroup_name": "igBASE_DM",
"items": [
{
"item_name": "BIRTHYR",
"value": "1976"
},
{
"item_name": "SEX",
"value": "M"
}
]
}
]
}
}
Response - Form is frozen (no actions happen), top level reported
{
"responseStatus": "FAILURE",
"errorMessage": "Form is frozen"
}
This endpoint is a singular call that can perform many actions at once, and conditionally peform actions should the state of the Subject data require such.
- The actions that can be performed cover these other endpoints:
- Upsert Forms (i.e. when Form repeats)
- Upsert Item Groups (i.e. when Item Groups in the Form repeat)
- Upsert Form Data (Items) (i.e. the actual setting of values to Items on the Form)
- Edit Submitted Form (i.e. to reopen Form currently in submitted state)
- Submit Form (i.e. submit the Form when finished, which runs study rules defined for the Form)
- Actions that do / can happen:
- If the Form is currently submitted, the Form is automatically reopened for edit (default)
- If the Form repeats in the study’s design, and does not yet exist, it will add that Form. Caveat: ‘Skipped’ sequences are invalid. For example, with 3 existing Forms (sequences 1,2,3), an attempt of sequence = 5 results in an error
- All necessary repeating Item Groups are added
- Change reasons are automatically applies if/when necessary when omitted in the request
- Optionally, the final step can be to submit the Form. This is the normal course to close the Form and have configured study rules run.
- Actions that do not happen:
- Any level above Form is not performed. For example, the Form lives in an Event which does not yet exist, i.e. the study design dictates dynamic add, user manual add, or other. The API attempt will fail in this case, the combination actions are only from Form down
- If a Form is locked, frozen, or part of locked Subject, locked site, locked study - the Form is not reopened, instead, the first step is a failure. These actions were / are specific human decisions, not to be circumvented via an API attempt.
- IMPORTANT: Rollbacks across multiple actions performed do not take place. For example, suppose the actions involved are Reopen Form, Add new Item Groups, set Item data, and Submit Form (4 in total). Then, suppose the Item updates fail (one or more). The end state is a reopened Form, with new Items groups added, i.e. partial success. A re-attempt of a corrected API request body would very likely pick up where the Form left off, i.e. this example the form is already open, Item Groups are already present, so the last two actions continue as normal.
- More than one Form attempted in the same API request
Endpoint
POST /api/{version}/app/cdm/forms/actions/setdata
Required Permissions
The following permissions are required to use the Combination Update Form Data API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
reopen |
Optional Whether to open the Form, if currently in submitted status. If omitted, true is assumed. | |
submit |
Optional Whether to submit the Form, as last step. If omitted, false is assumed. | |
change_reason |
Optional When omitted a default reason will be set, if a change reason required. | |
externally_owned |
Optional When omitted true is used, i.e. the site user cannot then further edit the value. Pass a false to re-enable the ability for a user to edit a previously read only Item. | |
study_country |
Name of the Study Country of the Subject | |
site |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. | |
subject |
Name of the Subject (ID / number) | |
eventgroup_name |
Study design name of the Event Group | |
eventgroup_sequence |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. | |
event_name |
Study design name of the Event | |
form_name |
Study design name of the Form | |
form_sequence |
Optional Specific form sequence (i.e., repeating form area) to perform on. If omitted, 1 is assumed. | |
itemgroup_name |
items_groups |
Study design name of the Item Group to be added within the Form |
itemgroup_sequence |
items_groups |
The specific Item Group sequence where the Item resides, Required even if a non-repeating Item Group. |
item_name |
items_groups/items |
Study design name for the Item |
value |
items_groups/items |
The value to set. See the table below for notes on Item types and value. |
unit_value |
items_groups/items |
Required when the Item is a unit codelist type. Omit otherwise. The value must be the unit codelist code, not label. |
Setting Data by Item Type
| Type | Example | Notes |
|---|---|---|
| Checkbox | "value": "true" |
i.e., checked |
| Checkbox | "value": "false" |
i.e., unchecked |
| Checkbox | "value": "" |
Same effect as false - Suggest use true / false |
| Checkbox | (any other value) | Error returned |
| Codelist | "value": "Y" |
e.g. "Yes" = "Y" in codelist definition |
| Codelist | "value": "" |
Empty / unset the selection |
| Codelist | (any other value, i.e., not a codelist code) | Error returned |
| URL | "value": "https://www.google.com" |
Set the value to a URL, on screen user can click to navigate to the URL, in another tab |
| URL | "value": "" |
Empty / unset the value |
| URL | (any value that does not start http or ftp) | Error returned |
| Text Field | "value": "Here is my text in the field" |
Set a value |
| Text Field | "value": "" |
Empty / unset the value |
| Text Field | (over max length) | Error returned |
| Numeric | "value": "3" |
E.g., a field with precision = 0 (no decimal portion) |
| Numeric | "value": "13.4" |
E.g., a decimal portion allowed field (precision > 0) |
| Numeric | "value": "" |
Empty / unset the value |
| Numeric | (not numeric or violates length or precision) | Error returned |
| Date | "value": "2022-06-01" |
E.g., to set June 1st, 2022 |
| Date | "value": "" |
Empty / unset the value |
| Date | "value": "2022-UN-UN" |
E.g., when the field allows unknowns in month and/or day. UN must be used in the appropriate portion |
| Date | (bad date, not of proper format, yyyy-MM-dd) | Error returned |
| DateTime | "value": "2022-06-01T12:30:00Z" |
Set the datetime in full, this will take on the 'on screen' value (not the normalized) |
| DateTime | "value": "" |
Empty / unset the value |
| DateTime | (bad date, not of proper format, yyyy-MM-ddTHH:mmZ) | Error returned |
| Time | "value": "12:30" |
Set the time value |
| Time | "value": "" |
Empty / unset the value |
| Time | (bad date, not of proper format, HH:mm) | Error returned |
| Unit Codelist | "value": "62.2", "unit_value": "in" |
E.g., 'in' is a valid code in the unit codelist definition. |
| Unit Codelist | "value": "" , "unit_value": "" |
Empty / unset the value |
| Unit Codelist | "value": "62.2" |
Error returned (must have both) |
| Unit Codelist | (any violation of length, precision, or invalid unit code) | Error returned |
| Item to Form Link | (example below and to the right) | IMPORTANT: The value portion for the Item must be included, but with no value for this Item Definition type. Only the information about the remote Form being linked to by the Item is indicated |
(example for Item to Form Link type of Item)
{
"item_name": "AE_LINK_TO_MH",
"value": "",
"item_to_form_link": {
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "MH",
"form_sequence": 3
}
}
:
Create Forms
Request - adding one new form
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/forms' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE"
}
]
}'
Response - adding one new form
{
"responseStatus": "SUCCESS",
"forms": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 1
}
]
}
Request - adding multiple forms
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/forms' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE"
},
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE"
}
]
}'
Response - adding multiple forms
{
"responseStatus": "SUCCESS",
"forms": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 2
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 3
}
]
}
Notes
- The action adds repeating Form instances, the next available sequence.
- The Maximum Repeats value of the Study design will be enforced when attempting.
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint..
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/forms
Required Permissions
The following permissions are required to use the Create Forms API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
forms |
Study design name of the Event where the Form resides |
form_name |
forms |
Study design name of the Form to add |
Upsert Forms
Request - adding one new form
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/forms' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE",
"form_sequence": 3
}
]
}'
Response - adding one new form
{
"responseStatus": "SUCCESS",
"forms": [
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 3
}
]
}
Request - adding multiple forms
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/forms' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE",
"form_sequence": 4
},
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"event_name": "evLOGS",
"form_name": "AE",
"form_sequence": 5
}
]
}'
Response - adding multiple forms
{
"responseStatus": "SUCCESS",
"forms": [
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 4
},
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egLOGS",
"eventgroup_sequence": 1,
"event_name": "evLOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 5
}
]
}
Notes
- The action adds repeating Form instances where they do not already exist, and skips requests where the Form already exists at that sequence.
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 100.
Endpoint
PUT /api/{version}/app/cdm/forms
Required Permissions
The following permissions are required to use the Upsert Forms API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
forms |
Study design name of the Event where the Form resides |
form_name |
forms |
Study design name of the Form to add |
form_sequence |
forms |
Required for the PUT version of this endpoint. The sequence of the Form to act on - created if does not exist, skipped if exists. |
Create Item Groups
Request - Add one item group, where form already has 3
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/itemgroups' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"itemgroups": [
{
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2"
}
]
}'
Response - Add one item group, where form already has 3
{
"responseStatus": "SUCCESS",
"itemgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 4
}
]
}
Request - Add two item groups, where form already has 4
curl -L -X POST 'https://my-vault.veevavault.com/api/{version}cdm/itemgroups' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"itemgroups": [
{
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2"
},
{
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2"
}
]
}'
Response - Add two item groups, where form already has 4
{
"responseStatus": "SUCCESS",
"itemgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 5
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "102",
"subject": "102-001",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 6
}
]
}
Notes
- The action adds repeating Item Group instances, the next available sequence.
- The Maximum Repeats value of the Study design will be enforced when attempting.
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/itemgroups
Required Permissions
The following permissions are required to use the Create Item Groups API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
itemgroups |
Name of the Study Country of the Subject |
site |
itemgroups |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
itemgroups |
Name of the Subject (ID / number) |
eventgroup_name |
itemgroups |
Study design name of the Event Group |
eventgroup_sequence |
itemgroups |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
itemgroups |
Study design name of the Event |
form_name |
itemgroups |
Study design name of the Form |
form_sequence |
itemgroups |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
itemgroups |
Study design name of the Item Group to be added within the Form |
Upsert Item Groups
Request - Add 2nd, 3rd, where 2nd already exists
curl -L -X PUT 'https://my-vault.veevavault.com/api/v24.1/cdm/itemgroups' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"itemgroups": [
{
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 2
},
{
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 3
}
]
}'
Response - Add 2nd, 3rd, where 2nd already exists
{
"responseStatus": "SUCCESS",
"itemgroups": [
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 2
},
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 3
}
]
}
Request - Add 3rd item group, where form currently has 1 (2 added, GAPS FILLED)
curl -L -X PUT 'https://my-vault.veevavault.com/api/v24.1/cdm/itemgroups' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"itemgroups": [
{
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"event_name": "evSCR",
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 3
}
]
}'
Response - Add 3rd item group, where form currently has 1 (2 added, GAPS FILLED)
{
"responseStatus": "SUCCESS",
"itemgroups": [
{
"responseStatus": "SUCCESS:CREATED",
"study_country": "United States",
"site": "102",
"subject": "SCR-0002",
"eventgroup_name": "egSCR",
"eventgroup_sequence": 1,
"event_name": "evSCR",
"event_sequence": 1,
"form_name": "IE",
"form_sequence": 1,
"itemgroup_name": "ig-IE2",
"itemgroup_sequence": 3
}
]
}
Notes
- The action adds repeating Item Group instances where they do not already exist, and skips requests where the Item Group already exists at that sequence.
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 100.
Endpoint
PUT /api/{version}/app/cdm/itemgroups
Required Permissions
The following permissions are required to use the Upsert Item Groups API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
itemgroups |
Name of the Study Country of the Subject |
site |
itemgroups |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
itemgroups |
Name of the Subject (ID / number) |
eventgroup_name |
itemgroups |
Study design name of the Event Group |
eventgroup_sequence |
itemgroups |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
itemgroups |
Study design name of the Event |
form_name |
itemgroups |
Study design name of the Form |
form_sequence |
itemgroups |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
itemgroups |
Study design name of the Item Group to be added within the Form |
itemgroup_sequence |
itemgroups |
Required, the specific sequence to have added. If the item group at that sequence already exists, the action is skipped |
Set Form Data (Items)
Request - Setting values for one of each type of item
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/items \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States"
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"form_name": "OE",
"form_sequence": 1,
"items": [
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CB",
"value": "true"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CL",
"value": "Y"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY",
"value": "2022-06-01"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "INTFIELD",
"value": "3"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DECIMAL",
"value": "5.2"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "URL_FIELD",
"value": "https://www.google.com"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "SHORTTXT",
"value": "Some text short"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload"
}
]
}
]
}'
Response - Setting values for one of each type of item
{
"responseStatus": "SUCCESS",
"items": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CB",
"value": "true",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CL",
"value": "Y",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY",
"value": "2022-06-01",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "INTFIELD",
"value": "3",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DECIMAL",
"value": "5.2",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "URL_FIELD",
"value": "https://www.google.com",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "SHORTTXT",
"value": "Some text short",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload",
"change_reason": "Action performed via the API",
"externally_owned": true
}
]
}
Notes
- Used to set (or update) data values on a Form.
- The endpoint does not create/upsert a Form that does not yet exist, nor open a Form for edit (if currently submitted).
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 25 Forms. Additionally, any one Form entry can only have at most 100 items within.
Endpoint
POST /api/{version}/app/cdm/items
Required Permissions
The following permissions are required to use the Set Form Data API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
forms |
Study design name of the Event |
form_name |
forms |
Study design name of the Form |
form_sequence |
forms |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
forms/items |
Study design name of the Item Group to be added within the Form |
itemgroup_sequence |
forms/items |
Optional The specific Item Group sequence where the Item resides. If omitted, 1 is assumed. It is strongly recommended to always include the sequence, even for non-repeating. |
value |
forms/items |
The value to set. See the table below for notes on Item types and value. |
unit_value |
forms/items |
Required when the Item is a unit codelist type. Omit otherwise. The value must be the unit codelist code, not label. |
change_reason |
forms/items |
Optional The reason for change of an Item value. For example: "Data updated by remote system" |
externally_owned |
forms/items |
Optional When omitted true is used, i.e. the site user cannot then further edit the value. Pass a false to re-enable the ability for a user to edit a previously read only Item. |
Upsert Form Data (Items)
Request - Setting values for one of each type of item
curl -X PUT https://my-vault.veevavault.com/api/v24.1/app/cdm/items \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"form_name": "OE",
"form_sequence": 1,
"items": [
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CB",
"value": "true"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CL",
"value": "Y"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY",
"value": "2022-06-01"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "INTFIELD",
"value": "3"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DECIMAL",
"value": "5.2"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "URL_FIELD",
"value": "https://www.google.com"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "SHORTTXT",
"value": "Some text short"
},
{
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload"
}
]
}
]
}'
Response - Setting values for one of each type of item
{
"responseStatus": "SUCCESS",
"items": [
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CB",
"value": "true",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "CL",
"value": "Y",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY",
"value": "2022-06-01",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEONLY_UNKS",
"value": "2022-UN-UN",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DATEANDTIME",
"value": "2022-06-01T12:30Z",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DT_WITH_UNKS",
"value": "2022-06-UNTUN:UNZ",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "INTFIELD",
"value": "3",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "DECIMAL",
"value": "5.2",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "UCLFIELD",
"value": "2.1",
"unit_value": "double_std",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "URL_FIELD",
"value": "https://www.google.com",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "SHORTTXT",
"value": "Some text short",
"change_reason": "Action performed via the API",
"externally_owned": true
},
{
"responseStatus": "SUCCESS:UPDATED",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"eventgroup_name": "egUNS",
"eventgroup_sequence": 3,
"event_name": "evUNS",
"event_sequence": 1,
"form_name": "OE",
"form_sequence": 1,
"itemgroup_name": "igOE",
"itemgroup_sequence": 1,
"item_name": "LONGTXT",
"value": "Some text in a long text field \n with a line break in the payload",
"change_reason": "Action performed via the API",
"externally_owned": true
}
]
}
Notes
- This endpoint is like Set Form Data (previous section, POST), but a later release (as PUT).
- Used to set (or update) data values on a Form.
- The endpoint does not create/upsert a Form that does not yet exist, nor open a Form for edit (if currently submitted).
- The current version of this endpoint returns
SUCCESS:UPDATED, even if no change was made on a specific Item. (i.e. a 'skip') - BEST PRACTICE - If also updating data on a Form, use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 25 Forms. Additionally, any one Form entry can only have at most 100 items within.
Setting Data by Item Type
| Type | Example | Notes |
|---|---|---|
| Checkbox | "value": "true" |
i.e., checked |
| Checkbox | "value": "false" |
i.e., unchecked |
| Checkbox | "value": "" |
Same effect as false - Suggest use true / false |
| Checkbox | (any other value) | Error returned |
| Codelist | "value": "Y" |
e.g. "Yes" = "Y" in codelist definition |
| Codelist | "value": "" |
Empty / unset the selection |
| Codelist | (any other value, i.e., not a codelist code) | Error returned |
| URL | "value": "https://www.google.com" |
Set the value to a URL, on screen user can click to navigate to the URL, in another tab |
| URL | "value": "" |
Empty / unset the value |
| URL | (any value that does not start http or ftp) | Error returned |
| Text Field | "value": "Here is my text in the field" |
Set a value |
| Text Field | "value": "" |
Empty / unset the value |
| Text Field | (over max length) | Error returned |
| Numeric | "value": "3" |
E.g., a field with precision = 0 (no decimal portion) |
| Numeric | "value": "13.4" |
E.g., a decimal portion allowed field (precision > 0) |
| Numeric | "value": "" |
Empty / unset the value |
| Numeric | (not numeric or violates length or precision) | Error returned |
| Date | "value": "2022-06-01" |
E.g., to set June 1st, 2022 |
| Date | "value": "" |
Empty / unset the value |
| Date | "value": "2022-UN-UN" |
E.g., when the field allows unknowns in month and/or day. UN must be used in the appropriate portion |
| Date | (bad date, not of proper format, yyyy-MM-dd) | Error returned |
| DateTime | "value": "2022-06-01T12:30:00Z" |
Set the datetime in full, this will take on the 'on screen' value (not the normalized) |
| DateTime | "value": "" |
Empty / unset the value |
| DateTime | (bad date, not of proper format, yyyy-MM-ddTHH:mmZ) | Error returned |
| Time | "value": "12:30" |
Set the time value |
| Time | "value": "" |
Empty / unset the value |
| Time | (bad date, not of proper format, HH:mm) | Error returned |
| Unit Codelist | "value": "62.2", "unit_value": "in" |
E.g., 'in' is a valid code in the unit codelist definition. |
| Unit Codelist | "value": "", "unit_value": "" |
Empty / unset the value |
| Unit Codelist | "value": "62.2" |
Error returned (must have both) |
| Unit Codelist | (any violation of length, precision, or invalid unit code) | Error returned |
Endpoint
PUT /api/{version}/app/cdm/items
Required Permissions
The following permissions are required to use the Upsert Form Data API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
forms |
Study design name of the Event |
form_name |
forms |
Study design name of the Form |
form_sequence |
forms |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
forms/items |
Study design name of the Item Group to be added within the Form |
itemgroup_sequence |
forms/items |
The specific Item Group sequence where the Item resides, Required even if a non-repeating Item Group. |
value |
forms/items |
The value to set. See the table below for notes on Item types and value. |
unit_value |
forms/items |
Required when the Item is a unit codelist type. Omit otherwise. The value must be the unit codelist code, not label. |
change_reason |
forms/items |
Optional The reason for change of an Item value. For example: "Data updated by remote system" |
externally_owned |
forms/items |
Optional When omitted true is used, i.e. the site user cannot then further edit the value. Pass a false to re-enable the ability for a user to edit a previously read only Item. |
Submit Forms
Request - Submit one form
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/forms/actions/submit \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"event_name": "Enrollment-Visit",
"form_name": "Randomization"
}
]
}'
Response - Submit one form
{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"event_name": "Enrollment-Visit",
"form_name": "Randomization"
}
]
}
Notes
- Used to perform the equivalent of the on screen Complete button.
- All study design Rules (of that Form) will be run as part of the action.
- BEST PRACTICE - If also updating data on a Form, use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/forms/actions/submit
Required Permissions
The following permissions are required to use the Submit Forms API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per study design. |
event_name |
forms |
Study design name of the Event |
form_name |
forms |
Study design name of the Form |
form_sequence |
forms |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
Reopen Submitted Forms
Request - Open one form for edit
curl -X POST https://cdms.vaultdev.com/api/{version}/app/cdm/forms/actions/edit \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"forms": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"event_name": "Enrollment-Visit",
"form_name": "Randomization",
"change_reason": "Transcription error"
}
]
}'
Response - Open one form for edit
{
"responseStatus": "SUCCESS",
"forms": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Enrollment-Visit",
"form_sequence": 1,
"change_reason": "Transcription error"
}
]
}
Notes
- Used to perform the equivalent of the on screen Edit Form button.
- BEST PRACTICE - Use the Combination Update Form Data endpoint, a more efficient approach than this legacy endpoint.
- The limit for actions in one request is 100.
Endpoint
POST /api/{version}/app/cdm/forms/actions/edit
Required Permissions
The following permissions are required to use the Reopen Submitted Forms API:
- API Access
- View Casebook
- Data Entry
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
forms |
Name of the Study Country of the Subject |
site |
forms |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
forms |
Name of the Subject (ID / number) |
eventgroup_name |
forms |
Study design name of the Event Group |
eventgroup_sequence |
forms |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per Study design. |
event_name |
forms |
Study design name of the Event |
form_name |
forms |
Study design name of the Form |
form_sequence |
forms |
Optional Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
change_reason |
forms |
The reason for change for editing the Form |
Queries
Queries are a method of review or error checking of entered data. There are two types of queries:
System queries generally originate from a Study design rule at the submit of a Form. (by API or end user). Vault creates these queries based on configured data validation rules in your Study.
Manual queries are those added by an end user, or by an API user. That is, they are not tied to a Study design rule / validation. Examples include CRA or Data Manager creating one with questions to the Site user, or ones created via Open Queries.
Learn more about queries in Clinical Data Help.
Retrieve Queries
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries?study_name=ABCP-2022-01_DEV1&study_country=United States&site=0101' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"queries": [
{
"id": "OPW000000003001",
"query_name": "VV-000003",
"manual": true,
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-003",
"eventgroup_name": "enrollment",
"eventgroup_sequence": 1,
"event_name": "enrollment_visit",
"event_sequence": 1,
"created_date": "2021-09-27T23:29:51Z",
"created_by": "Cordelia Hunter",
"messages": [
{
"id": "OPY000000005001",
"activity": "open__v",
"message": "Open query on Event",
"message_date": "2021-09-27T23:29:52Z",
"message_by": "Cordelia Hunter"
}
]
},
{
"id": "OPW000000004001",
"query_name": "VV-000004",
"manual": true,
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "common_forms",
"eventgroup_sequence": 1,
"event_name": "log_event",
"event_sequence": 1,
"form_name": "adverse_event",
"form_sequence": 1,
"itemgroup_name": "adverse_event_duration",
"itemgroup_sequence": 1,
"item_name": "aestart",
"created_date": "2021-09-27T23:33:30Z",
"created_by": "Cordelia Hunter",
"messages": [
{
"id": "OPY000000006001",
"activity": "open__v",
"message": "Open query on item",
"message_date": "2021-09-27T23:33:31Z",
"message_by": "Cordelia Hunter"
}
]
}
]
}
Notes
- Use this API to retrieve the existing Queries using Study Context.
- When the Query was added via a specific design rule, the
rule_definitionindicates the name of the rule that originated the Query. - Queries are added at the Item or Event level. For Event level, the Form ‘down’ information is of course omitted
- The respones includes a messages array with each comment/message appended to the Query. Reading top down in that array also reveals the progression of Query status, and by whom
- This endpoint is used to retrieve a list of queries for a Study. You can filter this list using one or more of the following:
- Study Country
- Site
- Subject
- Form Definition
- Status (of the Query)
- Queries added against specific Medical Coding Requests (see Retrieve Coding Queries)
- Last modified date/time of the Query
Endpoint
GET /api/{version}/app/cdm/queries?study_name={study_name}&...other filters here...
Required Permissions
The following permissions are required to use the Retrieve Queries API:
- API Access
- View Casebook
- View Query (if this feature is enabled in your Vault)
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
study_country |
Optional Name of the Study Country to filter to |
site |
Optional Name of the Site to filter to. Must include the study_country when using this. |
subject |
Optional Name (ID/number) to filter to. Must include site when using this. |
form_name |
Optional Study design name of a Form to filter to, e.g. all queries on the AE Form, across the Study. |
query_status |
Optional Current status of the queries to filter to. Values to use are one of open__v, answered__v, or closed__v. |
last_modified_date |
Optional Retrieve only Queries modified after this datetime. Use the format "yyyy-MM-ddTHH:mm:ssZ". |
id |
See next section: Retrieve Queries by ID (study context, by ID(s), or by coding definition are used, only one style) |
coding_item_definition |
See later section: Retrieve Coding Queries (study context, by ID(s), or by coding definition are used, only one style) |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Queries (as JSON array entries) with the following information:
| Array | Name | Description |
|---|---|---|
queries |
id |
Vault internal ID for the Query |
queries |
query_name |
Another Vault internal ID for the Query, this one is visible from the UI |
queries |
manual |
Whether the Query is classified System (false) or Manual (true) |
queries |
query_status |
Values will be one of open__v, answered__v, or closed__v |
queries |
study_country |
Name of the Study Country for the site of the Subject |
queries |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
queries |
subject |
Name (ID/number) of the Subject |
queries |
eventgroup_name |
Study design name for the Event Group |
queries |
eventgroup_sequence |
Sequence of the Event Group of the Event. If repeating Event Group the value increments starting at 1, 2.... If it does not repeat, then 1 is always returned |
queries |
event_name |
Study design name for a the Event |
queries |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level |
queries |
form_name |
(When attached to an Item only, omitted for Event queries) Study design name for a Form |
queries |
form_sequence |
(When attached to an Item only, omitted for Event queries) If the Form repeats per Study design, these increment starting at 1, 2.... If it does repeat, 1 is always returned. |
queries |
itemgroup_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item Group |
queries |
itemgroup_sequence |
(When attached to an Item only, omitted for Event queries) The specific Item Group sequence |
queries |
item_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item |
queries |
rule_definition |
When a Query originating from a Study design rule, that rule name. Omitted otherwise. |
queries |
coding_item_definition |
When a Query originating from Open Coding Queries, that medical coding definition name. Omitted otherwise. |
queries |
created_date |
The datetime that the Query was first created (UTC) |
queries |
created_by |
Name (Firstname Lastname) of the User who created the Query |
queries/messages |
id |
Vault internal ID of the Query message. These entries reading top to bottom (in the array) comprise the movement of statuses, plus comments of the Query |
queries/messages |
activity |
The Query status at that entry. Values will be one of open__v, answered__v, or closed__v |
queries/messages |
message |
The message at that Query update. Some activity entries do not include a specific message (e.g. close of an added Query) |
queries/messages |
message_date |
The datetime that this message/status change (UTC) |
queries/messages |
message_by |
Name (Firstname Lastname) of the User who moved the status / commented at this revision of the Query |
Retrieve Queries by ID
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries?study_name=ABCP-2022-01_DEV1&id=OPW000000003001' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"queries": [
{
"id": "OPW000000003001",
"query_name": "VV-000003",
"manual": true,
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-003",
"eventgroup_name": "enrollment",
"eventgroup_sequence": 1,
"event_name": "enrollment_visit",
"event_sequence": 1,
"created_date": "2021-09-27T23:29:51Z",
"created_by": "Cordelia Hunter",
"messages": [
{
"id": "OPY000000005001",
"activity": "open__v",
"message": "Open query on Event",
"message_date": "2021-09-27T23:29:52Z",
"message_by": "Cordelia Hunter"
}
]
}
]
}
Notes
- Use this API to retrieve the existing Queries of a specific ID (or IDs).
- The ID of the Query is returned on add via the API, or from Retrieve Queries.
- When the Query was added via a specific design rule, the
rule_definitionindicates the name of the rule that originated the Query. - Queries are added at the Item or Event level. For Event level, the Form ‘down’ information is of course omitted
- The respones includes a messages array with each comment/message appended to the Query. Reading top down in that array also reveals the progression of Query status, and by whom
Endpoint
GET /api/{version}/app/cdm/queries?study_name={study_name}&id={id}
Required Permissions
The following permissions are required to use the Retrieve Queries by ID API:
- API Access
- View Casebook
- View Query (if this feature is enabled in your Vault)
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
last_modified_date |
Optional Retrieve only Queries modified after this datetime. Use the format "yyyy-MM-ddTHH:mm:ssZ". |
id |
The Vault internal ID of the Query, or multiple in a comma-separated list. This parameter overrides all other parameters, except for study_name |
This API supports pagination, although unlikely to use paging when searching specific Queries by ID. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Queries (as JSON array entries) with the following information:
| Array | Name | Description |
|---|---|---|
queries |
id |
Vault internal ID for the Query |
queries |
query_name |
Another Vault internal ID for the Query, this one is visible from the UI |
queries |
manual |
Whether the query is classified System (false) or Manual (true) |
queries |
query_status |
Values will be one of open__v, answered__v, or closed__v |
queries |
study_country |
Name of the Study Country for the site of the Subject |
queries |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
queries |
subject |
Name (ID/number) of the Subject |
queries |
eventgroup_name |
Study design name for the Event Group |
queries |
eventgroup_sequence |
Sequence of the Event Group of the Event. If repeating Event Group the value increments starting at 1, 2.... If it does not repeat, then 1 is always returned |
queries |
event_name |
Study design name for a the Event |
queries |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level |
queries |
form_name |
(When attached to an Item only, omitted for Event queries) Study design name for a Form |
queries |
form_sequence |
(When attached to an Item only, omitted for Event queries) If the Form repeats per Study design, these increment starting at 1, 2.... If it does repeat, 1 is always returned. |
queries |
itemgroup_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item Group |
queries |
itemgroup_sequence |
(When attached to an Item only, omitted for Event queries) The specific Item Group sequence |
queries |
item_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item |
queries |
rule_definition |
When a Query originating from a Study design rule, that rule name. Omitted otherwise. |
queries |
coding_item_definition |
When a Query originating from Open Coding Queries, that medical coding definition name. Omitted otherwise. |
queries |
created_date |
The datetime that the Query was first created (UTC) |
queries |
created_by |
Name (Firstname Lastname) of the User who created the Query |
queries/messages |
id |
Vault internal ID of the Query message. These entries reading top to bottom (in the array) comprise the movement of statuses, plus comments of the Query |
queries/messages |
activity |
The Query status at that entry. Values will be one of open__v, answered__v, or closed__v |
queries/messages |
message |
The message at that Query update. Some activity entries do not include a specific message (e.g. close of an added Query) |
queries/messages |
message_date |
The datetime that this message/status change (UTC) |
queries/messages |
message_by |
Name (Firstname Lastname) of the User who moved the status / commented at this revision of the Query |
Open Queries
Request - Add one query, item level
curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/queries \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
"form_name": "F.DM",
"form_sequence": 1
"itemgroup_name": "IG.DEMOG",
"itemgroup_sequence": 1,
"item_name": "DOB",
"message": "This date does not match our records."
}
]
}'
Response - Add one query, item level
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000006001",
"query_name": "VV-000091",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
"form_name": "F.DM",
"form_sequence": 1,
"itemgroup_name": "IG.DM",
"itemgroup_sequence": 1,
"item_name": "DOB"
}
]
}
Request - Add two queries, item level and event level
curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/queries \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
"message": "Please double check the screening date entered"
},
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
"form_name": "F.DM",
"form_sequence": 1
"itemgroup_name": "IG.DEMOG",
"itemgroup_sequence": 1,
"item_name": "DOB",
"message": "This date does not match our records."
}
]
}'
Response - Add two queries, item level and event level
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000006001",
"query_name": "VV-000091",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
}
{
"responseStatus": "SUCCESS",
"id": "OPW000000006002",
"query_name": "VV-000091",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "EG.SCR",
"eventgroup_sequence": 1,
"event_name": "EV.SCR",
"form_name": "F.DM",
"form_sequence": 1,
"itemgroup_name": "IG.DM",
"itemgroup_sequence": 1,
"item_name": "DOB"
}
]
}
- The action is used to add a new Query to an Item of a Form, or Event of a specific Subject, by study context.
- WARNING: A locked Form will not allow the add a new Query, just as the EDC UI would disallow. Frozen Forms will accept new Queries added via the API.
- For further manipulation of Queries (answer, close, re-open), it's good practice to inspect the return on Query API actions, for the
idvalue. - The API user role must have permission to add Queries.
- The limit for actions in one request is 100.
- Other options exist to open a new Query, see also:
- Open Queries by Event ID - a Query added against a specific Event, when the Vault ID of that Event is known
- Open Queries by Item ID - a Query added against a specific Item, when the Vault ID of that Item is known
- Open Coding Query - a Query added against a specific Medical Coding Request
Endpoint
POST /api/{version}/app/cdm/queries
Required Permissions
The following permissions are required to use the Open Queries API:
- API Access
- Open Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
queries |
Name of the Study Country of the Subject |
site |
queries |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
queries |
Name of the Subject (ID / number) |
eventgroup_name |
queries |
Study design name of the Event Group |
eventgroup_sequence |
queries |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per Study design. |
event_name |
queries |
Study design name of the Event |
form_name |
queries |
Optional (required for Item queries) Study design name of the Form |
form_sequence |
queries |
Optional (required for Item queries) Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
queries |
Optional (required for Item queries) Study design name of the Item Group where the Item resides |
itemgroup_sequence |
queries |
Optional (required for Item queries) The specific Item Group sequence where the Item resides. If omitted, 1 is assumed. It is strongly recommended to always include the sequence, even for non-repeating. |
message |
queries |
The initial message of the Query |
Open Queries by Event ID
Request
curl -L -g -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/events/actions/openquery' \
-H 'Authorization: {SESSION_ID}' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"manual": false,
"queries": [
{
"id": "OPS00000001X001",
"message": "We ran screening at this site on Saturday, July 31."
}
]
}'
Response
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000003001",
"query_status": "open__v",
"event_id": "OPS00000001X001",
"query_name": "VV-000248",
"created_date": "2022-06-16T15:52:03Z",
"created_by": "Eric Emerton",
"messages": [
{
"id": "OPY000000012001",
"activity": "open__v",
"message": "We ran screening at this site on Saturday, July 31.",
"message_date": "2022-06-16T15:52:04Z",
"message_by": "Eric Emerton"
}
]
}
]
}
Notes
- The action is used to add a new Query to an Event of a specific Subject.
- WARNING: A locked Event will not allow the add a new Query, just as the EDC UI would disallow. Frozen Events will accept new Queries added via the API.
- For further manipulation of Queries (answer, close, re-open), it's good practice to inspect the return on Query API actions, for the
idvalue. - The API user role must have permission to add Queries.
- The limit for actions in one request is 500.
- Other options exist to open a new Query, see also:
- Open Queries - a Query added by specifying the context of the location (Study Country, Site, Subject, Event Group, etc.)
- Open Queries by Item ID - a Query added against a specific Item, when the Vault ID of that Item is known
- Open Coding Queries - a Query added against a specific Medical Coding Request
Endpoint
POST /api/{version}/app/cdm/events/actions/openquery
Required Permissions
The following permissions are required to use the Open Queries by Event ID API:
- API Access
- Open Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
manual |
Optional Whether the Query (or queries) should be considered manual. The default is true when omitted. A value of false implies the Query is added by a system, although not necessarily the EDC system. This value will be applied to all queries added in the request. | |
id |
queries |
The Vault internal ID of the Event to open the Query on. |
message |
queries |
The initial message of the Query |
Open Queries by Item ID
Request
curl -L -g -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/items/actions/openquery' \
-H 'Authorization: {SESSION_ID}' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"manual": false,
"queries": [
{
"id": "OPV000000000641",
"message": "This item value is out of range. Please correct."
}
]
}'
Response
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000004001",
"query_status": "open__v",
"item_id": "OPV000000000641"
"query_name": "VV-000249",
"created_date": "2022-07-13T15:52:03Z",
"created_by": "Eric Emerton",
"messages": [
{
"id": "OPY000000012001",
"activity": "open__v",
"message": "This item value is out of range. Please correct.",
"message_date": "2022-07-13T15:52:04Z",
"message_by": "Eric Emerton"
}
]
}
]
}
Notes
- The action is used to add a new Query to an Item (of a Form) of a specific Subject.
- WARNING: A locked Form will not allow the add a new Query, just as the EDC UI would disallow. Frozen Forms / Items will accept new Queries added via the API.
- For further manipulation of Queries (answer, close, re-open), it's good practice to inspect the return on Query API actions, for the
idvalue. - The API user role must have permission to add Queries.
- The limit for actions in one request is 500.
- Other options exist to open a new Query, see also:
- Open Queries - a Query added by specifying the context of the location (Study Country, Site, Subject, Event Group, etc.)
- Open Queries by Event ID - a Query added against a specific Event, when the Vault ID of that Event is known
- Open Coding Queries - a Query added against a specific Medical Coding Request
Endpoint
POST /api/{version}/app/cdm/items/actions/openquery
Required Permissions
The following permissions are required to use the Open Queries by Item ID API:
- API Access
- Open Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
manual |
Optional Whether the Query (or queries) should be considered manual. The default is true when omitted. A value of false implies the Query is added by a system, although not necessarily the EDC system. This value will be applied to all queries added in the request. | |
id |
queries |
The Vault internal ID of the Item to open the Query on. |
message |
queries |
The initial message of the Query |
Open Queries by Medical Coding Request
See the section, Open Coding Queries for these details.
Answer Queries
Request - Answer by Country/Site/Subject...
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/answer \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization",
"message": "This subject was enrolled late."
}
]
}'
Response - Answer by Country/Site/Subject...
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000000201",
"query_name": "VV-000002",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization"
}
]
}
Request - Answer by ID
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/answer' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"id": "OPW000000001001",
"message": "The screening date is correct."
}
]
}'
Response - Answer by ID
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000001001",
"query_name": "VV-000001",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1
}
]
}
Notes
- Used to answer a Query that is currently in the Open status, with a comment (required).
- The action moves the Query from the Open (
open__v) status into the Answered (answered__v) status. - A message is required.
- The limit for actions in one request is 100.
- There are two ways to answer a Query:
- By its internal Vault ID (returned in response on open Query, or from Retrieve Queries)
- By providing Study context to uniquely identify it (Study Country, Site, Subject, Event Group, Event, etc.)
Endpoint
POST /api/{version}/app/cdm/queries/actions/answer
Required Permissions
The following permissions are required to use the Answer Queries API:
- API Access
- Answer Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters (by Study Context)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
queries |
Name of the Study Country of the Subject |
site |
queries |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
queries |
Name of the Subject (ID / number) |
eventgroup_name |
queries |
Study design name of the Event Group |
eventgroup_sequence |
queries |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per Study design. |
event_name |
queries |
Study design name of the Event |
form_name |
queries |
Optional (required for Item queries) Study design name of the Form |
form_sequence |
queries |
Optional (required for Item queries) Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
queries |
Optional (required for Item queries) Study design name of the Item Group where the Item resides |
itemgroup_sequence |
queries |
Optional (required for Item queries) The specific Item Group sequence where the Item resides. If omitted, 1 is assumed. It is strongly recommended to always include the sequence, even for non-repeating. |
message |
queries |
The message at answer of the Query |
Body Parameters (By ID)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
id |
queries |
The Vault ID of the Query, found from response on open of the Query or from Retrieve Queries endpoint |
message |
queries |
The message at answer of the Query |
Close Queries
Request - Close by Country/Site/Subject...
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/close \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization",
"message": "This issue is now resolved. Query closed."
}
]
}'
Response - Close by Country/Site/Subject...
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000000201",
"query_name": "VV-000002",
"query_status": "closed__v",
"study_country": "United States",
"site": "101",
"subject": "42957928309475",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization"
}
]
}
Request - Close by ID
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/close' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"id": "OPW000000001001",
"message": "Closing query - verified that screening date is correct."
}
]
}'
Response - Close by ID
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000001001",
"query_name": "VV-000001",
"query_status": "closed__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1
}
]
}
Notes
- Used to close a Query that is currently in the Answered status, with a comment (optional).
- The action moves the Query from the Answered (
answered__v) status into the Closed (closed__v) status. - A message is not required, although is good practice.
- The limit for actions in one request is 100.
- There are two ways to close a Query:
- By providing Study context to uniquely identify it (Study Country, Site, Subject, Event Group, Event, etc.)
- By its internal Vault ID (returned in response on open Query, or from Retrieve Queries).
- As of v22.2, the close by ID style of this endpoint should be avoided, instead using the Close Queries (by ID / Batch) endpoint. The by ID style of this endpoint will be deprecated in a future release.
Endpoint
POST /api/{version}/app/cdm/queries/actions/close
Required Permissions
The following permissions are required to use the Close Queries API:
- API Access
- Close Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters (by Study Context)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
queries |
Name of the Study Country of the Subject |
site |
queries |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
queries |
Name of the Subject (ID / number) |
eventgroup_name |
queries |
Study design name of the Event Group |
eventgroup_sequence |
queries |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per Study design. |
event_name |
queries |
Study design name of the Event |
form_name |
queries |
Optional (required for Item queries) Study design name of the Form |
form_sequence |
queries |
Optional (required for Item queries) Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
queries |
Optional (required for Item queries) Study design name of the Item Group where the Item resides |
itemgroup_sequence |
queries |
Optional (required for Item queries) The specific Item Group sequence where the Item resides. If omitted, 1 is assumed. It is strongly recommended to always include the sequence, even for non-repeating. |
message |
queries |
Optional The message at close of the Query |
Body Parameters (By ID)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
id |
queries |
The Vault ID of the Query, found from response on open of the Query or from Retrieve Queries endpoint |
message |
queries |
Optional The message at close of the Query |
Close Queries (by ID / Batch)
Request - Close by ID (22R2 and later...)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/closebyid' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"id": "OPW000000001001",
"message": "Closing query - verified that screening date is correct."
},
{
"id": "OPW000000001002",
"message": "Closing query - added in error."
}
]
}'
Response - Close by ID (22R2 and later...)
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000001001",
"message": "Closing query - verified that screening date is correct.",
"query_status": "closed__v"
},
{
"responseStatus": "SUCCESS",
"id": "OPW000000001002",
"message": "Closing query - added in error.",
"query_status": "closed__v"
}
]
}
Notes
- Close a Query (or many) by ID.
- This endpoint replaces the By ID style of the Close Queries endpoint.
- The ID(s) of existing Queries can be found using the Retrieve Queries endpoint.
- The limit for actions in one request is 500.
Endpoint
POST /api/{version}/app/cdm/queries/actions/closebyid
Required Permissions
The following permissions are required to use the Close Queries API:
- API Access
- Close Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
id |
queries |
The Vault ID of the Query, found from response on open of the Query or from Retrieve Queries endpoint |
message |
queries |
Optional The message at close of the Query |
Reopen Queries
Request - Reopen by Country/Site/Subject...
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/reopen \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization",
"message": "Sign-in logs indicate that this subject was actually enrolled on the 20th. Reopening the query."
}
]
}'
Response - Reopen by Country/Site/Subject...
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000000201",
"query_name": "VV-000002",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Enrollment-Visit",
"eventgroup_sequence": 1,
"event_name": "Enrollment-Visit",
"event_sequence": 1,
"form_name": "Randomization",
"form_sequence": 1,
"itemgroup_name": "Randomization",
"itemgroup_sequence": 1,
"item_name": "Date-of-randomization"
}
]
}
Request - Reopen by ID
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries/actions/reopen' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"id": "OPW000000001001",
"message": "Reopening query: Received new information."
}
]
}'
Response - Reopen by ID
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPW000000001001",
"query_name": "VV-000001",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "screening",
"eventgroup_sequence": 1,
"event_name": "screening_visit",
"event_sequence": 1
}
]
}
Notes
- Used to reopen a Query that is currently in the Closed (
closed__v) status, with a comment (required). - The action moves the Query into the Open (
open__v) status. - The limit for actions in one request is 100.
- There are two ways to reopen a Query:
- By its internal Vault ID (returned in response on open Query, or from Retrieve Queries)
- By providing Study context to uniquely identify it (Study Country, Site, Subject, Event Group, Event, etc.)
Endpoint
POST /api/{version}/app/cdm/queries/actions/reopen
Required Permissions
The following permissions are required to use the Reopen Queries API:
- API Access
- Open Query
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters (by Study Context)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
study_country |
queries |
Name of the Study Country of the Subject |
site |
queries |
Name of the Site of the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
subject |
queries |
Name of the Subject (ID / number) |
eventgroup_name |
queries |
Study design name of the Event Group |
eventgroup_sequence |
queries |
Optional If omitted, 1 is assumed. Use this parameter if the Event Group is repeating, per Study design. |
event_name |
queries |
Study design name of the Event |
form_name |
queries |
Optional (required for Item queries) Study design name of the Form |
form_sequence |
queries |
Optional (required for Item queries) Specific Form sequence (i.e., repeating Form area) to perform on. If omitted, 1 is assumed. |
itemgroup_name |
queries |
Optional (required for Item queries) Study design name of the Item Group where the Item resides |
itemgroup_sequence |
queries |
Optional (required for Item queries) The specific Item Group sequence where the Item resides. If omitted, 1 is assumed. It is strongly recommended to always include the sequence, even for non-repeating. |
message |
queries |
The message at reopen of the Query |
Body Parameters (By ID)
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
id |
queries |
The Vault ID of the Query, found from response on open of the Query or from Retrieve Queries endpoint |
message |
queries |
The message at reopen of the Query |
Coding
A Medical Coding Request represents an Item that is being coded, e.g Severe Headache on an AE Form. These requests are added and updated upon the submission of the Form containing the Item. MedDRA and WHODrug (B3 or C3) versions are configured per Study design.
Retrieve Coding Requests
Request - 2 MedDRA coding requests, one coded, one not
curl --location --request GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/codingrequests?study_name=ABCP-2022-01_DEV1&coding_item_definition_id=V0T000000002001' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - 2 MedDRA coding requests, one coded, one not
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"codingrequests": [
{
"coding_item_definition_id": "V0T000000002001",
"form_coding_status": "active__v",
"form_type": "ae__v",
"dictionary_definition": "MedDRA",
"requests": [
{
"id": "V0V000000003001",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "My Term 1",
"start_date": "",
"stop_date": "",
"eventgroup_name": "LOGS",
"eventgroup_sequence": 1,
"event_name": "LOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 1,
"itemgroup_name": "ig-AE",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"coding_status": "open__v",
"last_modified_date": "2022-05-23T17:34:46Z",
"created_date": "2022-05-23T17:34:46Z",
"created_by": "System",
"other_properties": {
"My Label for SAE Form Uploaded": null
},
"seriousness": "Yes",
"severity": "",
"llt_code": "",
"llt": "",
"pt_code": "",
"pt": "",
"hlt_code": "",
"hlt": "",
"hlgt_code": "",
"hlgt": "",
"soc": "",
"soc_code": "",
"primary_path": ""
},
{
"id": "V0V000000003002",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "My AE 2",
"start_date": "2022-05-01T00:00:00Z",
"stop_date": "",
"eventgroup_name": "LOGS",
"eventgroup_sequence": 1,
"event_name": "LOGS",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 2,
"itemgroup_name": "ig-AE",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"coding_status": "coded__v",
"last_coded_date": "2022-06-02T18:35:03Z",
"last_modified_date": "2022-06-02T18:35:03Z",
"created_date": "2022-05-23T17:35:27Z",
"created_by": "System",
"other_properties": {
"My Label for SAE Form Uploaded": "true"
},
"seriousness": "No",
"severity": "",
"llt_code": "10027599",
"llt": "Migraine",
"pt_code": "10027599",
"pt": "Migraine",
"hlt_code": "10027603",
"hlt": "Migraine headaches",
"hlgt_code": "10019231",
"hlgt": "Headaches",
"soc": "Nervous system disorders",
"soc_code": "10029205",
"primary_path": "Y"
}
]
}
]
}
Request - 2 WHODrug coding requests, one coded, one not
curl --location --request GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/codingrequests?study_name=ABCP-2022-01_DEV1&coding_item_definition_id=V0T000000004001' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - 2 WHODrug coding requests, one coded, one not
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"codingrequests": [
{
"coding_item_definition_id": "V0T000000004001",
"form_coding_status": "active__v",
"form_type": "cm__v",
"dictionary_definition": "WHODrug C3",
"requests": [
{
"id": "V0V000000004001",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Aspirin",
"start_date": "",
"stop_date": "",
"eventgroup_name": "LOGS",
"eventgroup_sequence": 1,
"event_name": "LOGS",
"event_sequence": 1,
"form_name": "CM",
"form_sequence": 1,
"itemgroup_name": "ig-CM",
"itemgroup_sequence": 1,
"item_name": "CMTRT",
"coding_status": "coded__v",
"last_coded_date": "2022-06-02T20:02:26Z",
"last_modified_date": "2022-06-02T20:02:26Z",
"created_date": "2022-06-02T20:00:44Z",
"created_by": "System",
"other_properties": {},
"indication": "Headache",
"route": "Oral",
"drug_name": "Aspirin",
"drug_code": "00002701004",
"preferred_code": "00002701001",
"preferred_name": "Acetylsalicylic acid",
"atc4_code": "N02BA",
"atc4": "Salicylic acid and derivatives",
"atc3_code": "N02B",
"atc3": "OTHER ANALGESICS AND ANTIPYRETICS",
"atc2_code": "N02",
"atc2": "ANALGESICS",
"atc1_code": "N",
"atc1": "NERVOUS SYSTEM"
},
{
"id": "V0V000000004002",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Ibuprofen",
"start_date": "",
"stop_date": "",
"eventgroup_name": "LOGS",
"eventgroup_sequence": 1,
"event_name": "LOGS",
"event_sequence": 1,
"form_name": "CM",
"form_sequence": 2,
"itemgroup_name": "ig-CM",
"itemgroup_sequence": 1,
"item_name": "CMTRT",
"coding_status": "open__v",
"last_coded_date": "",
"last_modified_date": "2022-06-02T20:01:47Z",
"created_date": "2022-06-02T20:01:47Z",
"created_by": "System",
"other_properties": {},
"indication": "My indication",
"route": "Nasal",
"drug_name": "",
"drug_code": "",
"preferred_code": "",
"preferred_name": "",
"atc4_code": "",
"atc4": "",
"atc3_code": "",
"atc3": "",
"atc2_code": "",
"atc2": "",
"atc1_code": "",
"atc1": ""
}
]
}
]
}
Request - WHODrug requests, filtered to just those with coded or auto coded status
curl --location --request GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/codingrequests?study_name=ABCP-2022-01_DEV1&coding_item_definition_id=V0T000000004001&coding_status=coded__v,autocoded__v' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - WHODrug requests, filtered to just those with coded or auto coded status
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 1,
"total": 1
},
"codingrequests": [
{
"coding_item_definition_id": "V0T000000004001",
"form_coding_status": "active__v",
"form_type": "cm__v",
"dictionary_definition": "WHODrug C3",
"requests": [
{
"id": "V0V000000004001",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Aspirin",
"start_date": "",
"stop_date": "",
"eventgroup_name": "LOGS",
"eventgroup_sequence": 1,
"event_name": "LOGS",
"event_sequence": 1,
"form_name": "CM",
"form_sequence": 1,
"itemgroup_name": "ig-CM",
"itemgroup_sequence": 1,
"item_name": "CMTRT",
"coding_status": "coded__v",
"last_coded_date": "2022-06-02T20:02:26Z",
"last_modified_date": "2022-06-02T20:02:26Z",
"created_date": "2022-06-02T20:00:44Z",
"created_by": "System",
"other_properties": {},
"indication": "Headache",
"route": "Oral",
"drug_name": "Aspirin",
"drug_code": "00002701004",
"preferred_code": "00002701001",
"preferred_name": "Acetylsalicylic acid",
"atc4_code": "N02BA",
"atc4": "Salicylic acid and derivatives",
"atc3_code": "N02B",
"atc3": "OTHER ANALGESICS AND ANTIPYRETICS",
"atc2_code": "N02",
"atc2": "ANALGESICS",
"atc1_code": "N",
"atc1": "NERVOUS SYSTEM"
}
]
}
]
}
Request - MedDRA requests, including external suggestions - one request with two suggestions, the other none
curl --location --request GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/codingrequests?study_name=ABCP-2022-01_DEV1&coding_item_definition_id=V0T000000004001&include_external_suggestions=true
' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - MedDRA requests, including external suggestions - one request with two suggestions, the other none
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"codingrequests": [
{
"coding_item_definition_id": "V0R00000003K001",
"form_coding_status": "active__v",
"form_type": "ae__v",
"dictionary_definition": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"requests": [
{
"id": "V0T000000027001",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Stroke",
"start_date": "",
"stop_date": "",
"eventgroup_name": "egMain",
"eventgroup_sequence": 1,
"event_name": "evMIN",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 1,
"itemgroup_name": "igAE",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"coding_status": "autocoded__v",
"last_coded_date": "2023-04-24T18:40:48Z",
"last_modified_date": "2023-04-24T18:40:48Z",
"created_date": "2023-04-24T18:40:46Z",
"created_by": "System",
"other_properties": {},
"seriousness": "",
"severity": "",
"soc_code": "10029205",
"soc": "Nervous system disorders",
"hlgt_code": "10007963",
"hlgt": "Central nervous system vascular disorders",
"hlt_code": "10007948",
"hlt": "Central nervous system haemorrhages and cerebrovascular accidents",
"pt_code": "10008190",
"pt": "Cerebrovascular accident",
"llt_code": "10042244",
"llt": "Stroke",
"primary_path": "Y",
"external_suggestions": [
{
"external_definition_name": "my_medcdr_company",
"external_id": "123456-ABC",
"relevance_score": 5,
"external_version": "APR-2023v1.1",
"soc_code": "10029205",
"soc": "Nervous system disorders",
"hlgt_code": "10019231",
"hlgt": "Headaches",
"hlt_code": "10027603",
"hlt": "Migraine headaches",
"pt_code": "10027599",
"pt": "Migraine",
"llt_code": "10027599",
"llt": "Migraine",
"primary_path": "Y"
},
{
"external_definition_name": "my_medcdr_company",
"external_id": "123457-ABC",
"relevance_score": 10,
"external_version": "APR-2023v1.1",
"soc_code": "10029205",
"soc": "Nervous system disorders",
"hlgt_code": "10007963",
"hlgt": "Central nervous system vascular disorders",
"hlt_code": "10007948",
"hlt": "Central nervous system haemorrhages and cerebrovascular accidents",
"pt_code": "10008190",
"pt": "Cerebrovascular accident",
"llt_code": "10042244",
"llt": "Stroke",
"primary_path": "Y"
}
]
},
{
"id": "V0T000000027002",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Stroke",
"start_date": "",
"stop_date": "",
"eventgroup_name": "egMain",
"eventgroup_sequence": 1,
"event_name": "evMIN",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 3,
"itemgroup_name": "igAE",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"coding_status": "open__v",
"last_coded_date": null,
"last_modified_date": "2023-04-24T18:40:48Z",
"created_date": "2023-04-24T18:40:46Z",
"created_by": "System",
"other_properties": {},
"seriousness": "",
"severity": "",
"soc_code": "",
"soc": "",
"hlgt_code": "",
"hlgt": "",
"hlt_code": "",
"hlt": "",
"pt_code": "",
"pt": "",
"llt_code": "",
"llt": "",
"primary_path": "",
"external_suggestions": []
}
]
}
]
}
Request - MedDRA requests, including external suggestions, but only those lacking suggestions
curl --location --request GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/codingrequests?study_name=ABCP-2022-01_DEV1&coding_item_definition_id=V0T000000004001&include_external_suggestions=true&has_external_suggestions=false
' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - MedDRA requests, including external suggestions, but only those lacking suggestions
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 1,
"total": 1
},
"codingrequests": [
{
"coding_item_definition_id": "V0R00000003K001",
"form_coding_status": "active__v",
"form_type": "ae__v",
"dictionary_definition": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"requests": [
{
"id": "V0T000000027002",
"study_country": "United States",
"site": "101",
"subject": "SCR-0001",
"verbatim": "Stroke",
"start_date": "",
"stop_date": "",
"eventgroup_name": "egMain",
"eventgroup_sequence": 1,
"event_name": "evMIN",
"event_sequence": 1,
"form_name": "AE",
"form_sequence": 3,
"itemgroup_name": "igAE",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"coding_status": "open__v",
"last_coded_date": null,
"last_modified_date": "2023-04-24T18:40:48Z",
"created_date": "2023-04-24T18:40:46Z",
"created_by": "System",
"other_properties": {},
"seriousness": "",
"severity": "",
"soc_code": "",
"soc": "",
"hlgt_code": "",
"hlgt": "",
"hlt_code": "",
"hlt": "",
"pt_code": "",
"pt": "",
"llt_code": "",
"llt": "",
"primary_path": "",
"external_suggestions": []
}
]
}
]
}
Notes
- Retrieve a list of Medical Coding Requests for a Form.
- For each request, coding status, definition information, and the assigned code (if any) are returned.
- The parameter to get existing Medical Coding Requests of a Form (
coding_item_definition_id) can be found by first using the Retrieve Coding Definitions API endpoint.
Endpoint
GET /api/{version}/app/cdm/coder/codingrequests
Required Permissions
The following permissions are required to use the Retrieve Coding Requests API:
- API Access
- View Code
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
coding_item_definition_id |
Specific medical coding definition of requests to return. The value is part of the Study design, and can be found using Retrieve Coding Definitions, specifically the coding_item_definition_id in that response. |
study_country |
Optional Name of the Study Country to filter the return on. |
site |
Optional Name of the Site to filter to. One must include the study_country when including. |
subject |
Optional Name (ID/number) to filter to. One must include site when including. |
last_modified_date |
Optional Retrieve only requests modified after this datetime. Use the format "yyyy-MM-ddTHH:mm:ssZ". |
coding_status |
Optional Retrieve only those requests of certain status (or statuses, if multiple, use comma separated value). Valid status values are - open__v, autocoded__v, uncoded__v, noncurrent__v, coded__v, rejected__v, pending_approval__v, not_to_be_coded__v, better_code_found__v, or updated__v. |
include_external_suggestions |
Optional Boolean value, whether or not to include any external suggestions to the requests. Default when omitted is false. Also note that the Study must also be configured with an external suggestion definition to return this information in the response. |
has_external_suggestions |
Optional Boolean value, when included, will filter the return to just those requests with external suggestions (true) in place, or not (false). When omitted, there is no filter one way or the other. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Medical Coding Requests (as JSON array entries) with the following information:
| Array | Name | Description |
|---|---|---|
codingrequests |
coding_item_definition_id |
Vault internal ID for the Study definition of the coding Item. (pre v22.3, this was coding_item_definition, now renamed to be in line with the Retrieve Coding Definitions API |
codingrequests |
form_coding_status |
The status of the Study definition of the coding at the Form (of the Item being coded). Formerly named form_status (pre v22.3), but clarified in name for which status regarding the definition. If the coding of the Study is not fully setup, a return here of needs_dictionary__v is likely. |
codingrequests |
form_type |
The coding definition type of Form, e.g. 'AE'. This is the picklist name (e.g. ae__v, cm__v, other__v) |
codingrequests |
dictionary_definition |
The type of coding at that location, e.g. WHODrug B3, WHODrug C3, or MedDRA |
codingrequests/requests |
id |
The Vault internal ID of the Medical Coding Request. Use this value when opening a Query on the coding request (Open Coding Queries) |
codingrequests/requests |
study_country |
Name of the Study Country for the site of the Subject |
codingrequests/requests |
site |
Name of the Site for the Subject (NOTE: Study Country to also be returned in a future release. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
codingrequests/requests |
subject |
Name (ID/number) of the Subject |
codingrequests/requests |
verbatim |
The current value in the Item being coded, e.g. Severe Headache |
codingrequests/requests |
start_date |
The value in the field mapped to start date (on the Form of the Item being coded) |
codingrequests/requests |
end_date |
The value in the field mapped to end date (on the Form of the Item being coded) |
codingrequests/requests |
eventgroup_name |
Study design name for Event Group |
codingrequests/requests |
event_sequence |
The sequence of the Event Group |
codingrequests/requests |
event_name |
Study design name for the Event (pre v22.3, this was the label and event, changed for consistency with other endpoints) |
codingrequests/requests |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level. |
codingrequests/requests |
form_name |
Study design name for the Form (pre v22.3, this was the label and form, changed for consistency with other endpoints) |
codingrequests/requests |
form_sequence |
The specific Form sequence |
codingrequests/requests |
itemgroup_name |
Study design name for Item Group (pre v22.3, this was the label and item_group, changed for consistency with other endpoints) |
codingrequests/requests |
itemgroup_sequence |
The specific Item Group sequence (pre v22.3, item_group_sequence, changed for consistency with other endpoints) |
codingrequests/requests |
item_name |
Study design name for the Item (pre v22.3, this was the label and item, changed for consistency with other endpoints) |
codingrequests/requests |
coding_status |
The current status of the coding request, e.g. open__v, coded__v, etc. (pre v22.3, this was the label, changed for consistency with other endpoints) |
codingrequests/requests |
last_coded_by_third_party |
The datetime that the request last had coding assigned / selected / auto coded by an external system from EDC (UTC). Omitted when not set - a future release of EDC will support 3rd party coding. |
codingrequests/requests |
last_coded_date |
The datetime that the request last had coding assigned / selected / auto coded (UTC) |
codingrequests/requests |
assigned_to_third_party |
If coding from another system involved, who in that other system the request is assigned. Omitted when not set - a future release of EDC will support 3rd party coding. |
codingrequests/requests |
last_modified_date |
The datetime that the request was last modified in any way (UTC) |
codingrequests/requests |
created_date |
The datetime that the request was first created (UTC) |
codingrequests/requests |
created_by |
Name (First Last) of the User who created the request |
codingrequests/requests |
other_properties |
If study design uses, a set of label/value pairs (JSON array entries), i.e., other values on the Form |
codingrequests/requests |
seriousness |
(MedDRA only) If the study design maps an Item on that same Form for seriousness, the value in that Item |
codingrequests/requests |
severity |
(MedDRA only) If the study design maps an Item on that same Form for severity, the value in that Item |
codingrequests/requests |
llt_code |
(MedDRA only) - numeric value of coding assignment at this level |
codingrequests/requests |
llt |
(MedDRA only) - text value of coding assignment at this level |
codingrequests/requests |
pt_code |
(MedDRA only)- numeric value of coding assignment at this level |
codingrequests/requests |
pt |
(MedDRA only)- text value of coding assignment at this level |
codingrequests/requests |
hlt_code |
(MedDRA only)- numeric value of coding assignment at this level |
codingrequests/requests |
hlt |
(MedDRA only)- text value of coding assignment at this level |
codingrequests/requests |
hlgt_code |
(MedDRA only)- numeric value of coding assignment at this level |
codingrequests/requests |
hlgt |
(MedDRA only)- text value of coding assignment at this level |
codingrequests/requests |
soc_code |
(MedDRA only)- numeric value of coding assignment at this level |
codingrequests/requests |
soc |
(MedDRA only)- text value of coding assignment at this level |
codingrequests/requests |
primary_path |
(MedDRA only) Values of Y or N only. If multiple paths involved for the coding assignment, whether it is the primary path (or not) |
codingrequests/requests |
indication |
(WHODrug only) If the study design maps an Item on that same Form for indication, the value in that Item |
codingrequests/requests |
route |
(WHODrug only) If the study design maps an Item on that same Form for route, the value in that Item |
codingrequests/requests |
drug_name |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
drug_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
preferred_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
preferred_name |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc4_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc4 |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc3_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc3 |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc2_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc2 |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc1_code |
(WHODrug only) Property from the coding that is assigned |
codingrequests/requests |
atc1 |
(WHODrug only) Property from the coding that is assigned |
Retrieve Coding Queries
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/queries?study_name=ABCP-2022-01_DEV1&coding_item_definition=V0R000000006001,V0R000000007001' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"queries": [
{
"id": "OPW000000000201"
"query_name": "VV-000002",
"manual": true,
"created_date": "2019-11-21T22:40:49Z",
"created_by": "John Doe",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Common Logs",
"eventgroup_sequence": 1,
"event_name": "Common Logs",
"event_sequence": 1,
"form_name": "Adverse Event",
"form_sequence": 1,
"itemgroup_name": "Adverse Event",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"rule_definition": "DM_001",
"coding_item_definition": "V0R000000006001",
"messages": [
{
"id": "OPY000000000301",
"activity": "open__v",
"message": "Can you relook at this verbatim?",
"message_date": "2019-11-21T22:40:50Z",
"message_by": "John Doe"
},
{
"id": "OPY000000000302",
"activity": "answered__v",
"message": "I updated it, take another look.",
"message_date": "2019-11-21T22:41:50Z",
"message_by": "Jane Doe"
},
{
"id": "OPY000000000303",
"activity": "closed__v",
"message": "Perfect, thanks.",
"message_date": "2019-11-21T22:42:50Z",
"message_by": "John Doe"
}
]
},
{
"id": "OPW000000000801",
"query_name": "VV-000002",
"manual": true,
"created_date": "2019-11-21T22:40:49Z",
"created_by": "John Doe",
"query_status": "open__v",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "Common Logs",
"eventgroup_sequence": 1,
"event_name": "Common Logs",
"event_sequence": 1,
"form_name": "Adverse Event",
"form_sequence": 1,
"itemgroup_name": "Adverse Event",
"itemgroup_sequence": 1,
"item_name": "AETERM",
"rule_definition": "DM_002",
"coding_item_definition": "V0R000000007001"
"messages": [
{
"id": "OPY000000000301",
"activity": "open__v",
"message": "Can you relook at this verbatim?",
"message_date": "2019-11-21T22:40:50Z",
"message_by": "John Doe"
},
{
"id": "OPY000000000302",
"activity": "answered__v",
"message": "I updated it, take another look.",
"message_date": "2019-11-21T22:41:50Z",
"message_by": "Jane Doe"
},
{
"id": "OPY000000000303",
"activity": "closed__v",
"message": "Perfect, thanks.",
"message_date": "2019-11-21T22:42:50Z",
"message_by": "John Doe"
}
]
}
]
}
Notes
- Use this API to retrieve the existing Queries using medical coding definitions, i.e. Queries created using Open Coding Queries.
- Queries opened any other way are not part of this response
- For the
coding_item_definitionparameter, the appropriate value can be found using Retrieve Coding Definitions, it'scoding_item_definition_idvalue returned. - The response includes a messages array with each comment/message appended to the Query. Reading top down in that array also reveals the progression of Query status, and by whom
Endpoint
GET /api/{version}/app/cdm/queries?study_name={study_name}&coding_item_definition={coding_item_definition}
Required Permissions
The following permissions are required to use the Retrieve Coding Queries API:
- API Access
- View Casebook
- View Code
- View Query (if this feature is enabled in your Vault)
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
coding_item_definition |
Specific medical coding definition (use comma-separated for multiple) to filter against. That is, queries added using Open Coding Queries against those definition(s). The value(s) are part of the Study design, and can be found using Retrieve Coding Definitions, values from coding_item_definition_id in that response. |
last_modified_date |
Optional Retrieve only Queries modified after this datetime. Use the format "yyyy-MM-ddTHH:mm:ssZ". |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Queries (as JSON array entries) with the following information:
| Array | Name | Description |
|---|---|---|
queries |
id |
Vault internal ID for the Query |
queries |
query_name |
Another Vault internal ID for the Query, this one is visible from the UI |
queries |
manual |
Whether the query is classified System (false) or Manual (true) |
queries |
query_status |
Values will be one of open__v, answered__v, or closed__v |
queries |
study_country |
Name of the Study Country for the site of the Subject |
queries |
site |
Name of the Site for the Subject. Name here refers to the Name field in Vault. It is alphanumeric and may consist of numbers, letters, or both. |
queries |
subject |
Name (ID/number) of the Subject |
queries |
eventgroup_name |
Study design name for the Event Group |
queries |
eventgroup_sequence |
Sequence of the Event Group of the Event. If repeating Event Group the value increments starting at 1, 2.... If it does not repeat, then 1 is always returned |
queries |
event_name |
Study design name for a the Event |
queries |
event_sequence |
Currently, always a 1. If the Event within a repeating Event Group the sequence increments at the evengroup_sequence level |
queries |
form_name |
(When attached to an Item only, omitted for Event queries) Study design name for a Form |
queries |
form_sequence |
(When attached to an Item only, omitted for Event queries) If the Form repeats per Study design, these increment starting at 1, 2.... If it does repeat, 1 is always returned. |
queries |
itemgroup_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item Group |
queries |
itemgroup_sequence |
(When attached to an Item only, omitted for Event queries) The specific Item Group sequence |
queries |
item_name |
(When attached to an Item only, omitted for Event queries) Study design name of the Item |
queries |
rule_definition |
When a Query originating from a Study design rule, that rule name. Omitted otherwise. |
queries |
coding_item_definition |
When a Query originating from Open Coding Queries, that medical coding definition name. Omitted otherwise. |
queries |
created_date |
The datetime that the Query was first created (UTC) |
queries |
created_by |
Name (Firstname Lastname) of the User who created the Query |
queries/messages |
id |
Vault internal ID of the Query message. These entries reading top to bottom (in the array) comprise the movement of statuses, plus comments of the Query |
queries/messages |
activity |
The Query status at that entry. Values will be one of open__v, answered__v, or closed__v |
queries/messages |
message |
The message at that Query update. Some activity entries do not include a specific message (e.g. close of an added Query) |
queries/messages |
message_date |
The datetime that this message/status change (UTC) |
queries/messages |
message_by |
Name (Firstname Lastname) of the User who moved the status / commented at this revision of the Query |
Open Coding Queries
Request
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/openquery \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"queries": [
{
"coding_request": "V0T00000000R001",
"message": "Can you relook at this verbatim?"
},
{
"coding_request": "V0T00000000R002",
"message": "Can you relook at this verbatim?"
}
]
}'
Response
{
"responseStatus": "SUCCESS",
"queries": [
{
"responseStatus": "SUCCESS",
"id": "OPM000000000502",
"query_name": "VV-000002",
"query_status": "open__v",
"coding_request": "V0T00000000R001"
},
{
"responseStatus": "SUCCESS",
"id": "OPM000000000502",
"query_name": "VV-000002",
"query_status": "open__v",
"coding_request": "V0T00000000R002"
}
]
}
Notes
- The action is used to add a new Query to an Item who is tagged with a specific Medical Coding Request.
- The
idof the Medical coding Request is used to add the Query (Item that the coding request is tied to) - WARNING: A locked Form will not allow the add a new Query, just as the EDC UI would disallow. Frozen Forms / Items will accept new Queries added via the API.
- For further manipulation of Queries (answer, close, re-open), it's good practice to inspect the return on Query API actions, for the
idvalue. - The API user role must have permission to add Queries.
- The limit for actions in one request is 100.
- Other options exist to open a new Query, see also:
- Open Queries - a Query added by specifying the context of the location (Study Country, Site, Subject, Event Group, etc.)
- Open Queries by Event ID - a Query added against a specific Event, when the Vault ID of that Event is known
- Open Queries by Item ID - a Query added against a specific Item, when the Vault ID of that Item is known
Endpoint
POST /api/{version}/app/cdm/coder/actions/openquery
Required Permissions
The following permissions are required to use the Open Coding Queries API:
- API Access
- View Casebook
- Open Query
- View Code
The CDMS API Read Write grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
coding_request |
queries |
The Vault internal ID of the Medical Coding Request to open the Query on. This request was added on submit of a Form that contains an Item being coded in Clinical Data Coder. The value to use is the id found from Retrieve Coding Requests response. |
message |
queries |
The initial message of the Query |
Set Coding Suggestions
Request - MedDRA suggestions
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/setcodingsuggestions \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"id": "V0V000000003002",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Severe Headache",
"external_suggestions": [
{
"external_id": "123456-ABC",
"relevance_score": 5,
"external_version": "JAN-2022v1.1",
"soc": "Nervous system disorders",
"soc_code": "10029205",
"hlgt_code": "10019231",
"hlgt": "Headaches",
"hlt_code": "10027603",
"hlt": "Migraine headaches",
"pt_code": "10027599",
"pt": "Migraine",
"llt_code": "10027599",
"llt": "Migraine",
"primary_path": "Y"
},
{
"external_id": "123457-ABC",
"relevance_score": 4,
"external_version": "JAN-2022v1.1",
:
... etc...
:
},
:
: etc.. 5 suggestions for this coding request
:
]
},
{
"id": "V0V000000003003",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Back pain",
"external_suggestions": [
:
: etc.. 3 suggestions for this coding request here...
:
]
}
]
}'
Response - MedDRA suggestions
{
"responseStatus": "SUCCESS",
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"responseStatus": "SUCCESS",
"id": "V0V000000003002",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Severe Headache",
"external_suggestions": 5
},
{
"responseStatus": "SUCCESS",
"id": "V0V000000003003",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Back pain",
"external_suggestions": 3
}
}
Request - WHODrug suggestions
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/setcodingsuggestions \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"id": "V0V000000003002",
"dictionary_release": "GLOBALC3Mar19",
"verbatim": "Aspirin",
"indication": "Headache",
"route": "Oral",
"external_suggestions": [
{
"external_id": "1234345-XYZ",
"relevance_score": 100,
"external_version": "JAN-2022v1.1",
"drug_name": "Aspirin",
"drug_code": "00002701004",
"preferred_code": "00002701001",
"preferred_name": "Acetylsalicylic acid",
"atc1_code": "N",
"atc1": "NERVOUS SYSTEM"
"atc2_code": "N02",
"atc2": "ANALGESICS",
"atc3_code": "N02B",
"atc3": "OTHER ANALGESICS AND ANTIPYRETICS",
"atc4_code": "N02BA",
"atc4": "Salicylic acid and derivatives",
}
{
"external_id": "1234345-XYZ",
"relevance_score": 95,
"external_version": "JAN-2022v1.1",
... etc...
},
:
: etc.. 5 suggestions for this coding request
:
]
},
{
"id": "V0V000000003003",
"dictionary_release": "GLOBALC3Mar19",
"verbatim": "Ibuprofen",
"indication": "Joint Pain",
"route": "Oral",
"external_suggestions": [
:
: etc.. 3 suggestions for this coding request here...
:
]
}
]
}'
Response - WHODrug suggestions
{
"responseStatus": "SUCCESS",
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"responseStatus": "SUCCESS",
"id": "V0V000000003002",
"verbatim": "Aspirin",
"indication": "Headache",
"route": "Oral",
"dictionary_release": "GLOBALC3Mar19",
"external_suggestions": 5
},
{
"responseStatus": "SUCCESS",
"id": "V0V000000003003",
"verbatim": "Ibuprofen",
"indication": "Joint Pain",
"route": "Oral",
"dictionary_release": "GLOBALC3Mar19",
"external_suggestions": 3
}
}
Request - Remove suggestions from a coding request
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/setcodingsuggestions \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"id": "V0V000000003002",
"dictionary_release": "GLOBALC3Mar19",
"external_suggestions": []
}
]
}'
Response - Remove suggestions from a coding request
{
"responseStatus": "SUCCESS",
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"responseStatus": "SUCCESS",
"id": "V0V000000003002",
"verbatim": "Aspirin",
"indication": "Headache",
"route": "Oral",
"dictionary_release": "GLOBALC3Mar19",
"external_suggestions": 0
}
}
Request - Top level failure (study not configured)
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/setcodingsuggestions \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here_bad_name",
"requests": [
{
"id": "V0V000000003002",
"dictionary_release": "GLOBALC3Mar19",
"external_suggestions": [
:
...etc..
:
]
},
:
etc.. more requests and suggestions
:
]
}'
Response - Top level failure (study not configured)
{
"responseStatus": "FAILURE",
"errorMessage": "[External Definition Name] with name [API_company_name_here_bad_name] not found in _Study_ configuration for external suggestions"
}
Request - Entry level failure (one succeeds, one fails)
$ curl -X POST \
https://my-vault.veevavault.com/api/v24.1/app/cdm/coder/actions/setcodingsuggestions \
-H "Authorization: {SESSION_ID}" \
-H "Accept: application/json" \
-H "Content-Type: application/json" \
-d '
{
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "API_company_name_here",
"requests": [
{
"id": "V0V000000003002",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Severe Headache",
"external_suggestions": [
{
"external_id": "123456-ABC",
"relevance_score": 5,
"external_version": "JAN-2022v1.1",
"soc": "Nervous system disorders",
"soc_code": "10029205",
"hlgt_code": "10019231",
"hlgt": "Headaches",
"hlt_code": "10027603",
"hlt": "Migraine headaches",
"pt_code": "10027599",
"pt": "Migraine",
"llt_code": "10027599",
"llt": "Migraine",
"primary_path": "Y"
}
{
"external_id": "123457-ABC",
"relevance_score": 4,
"external_version": "JAN-2022v1.1",
... etc...
},
{
... etc... other suggestions
}
]
},
{
"id": "V0V000000003002",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Back pain",
"external_suggestions": [
:
: etc.. 3 suggestions for this coding request here... but verbatim is stale / out of date
:
]
}
]
}'
Response - Entry level failure (one succeeds, one fails)
{
"responseStatus": "SUCCESS",
"study_name": "ABCP-2022-01_DEV1",
"external_definition_name": "My Company Name",
"requests": [
{
"responseStatus": "SUCCESS",
"id": "V0V000000003002",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Severe Headache",
"external_suggestions": 5
},
{
"responseStatus": "FAILURE",
"errorMessage": "The verbatim submitted with the suggestions is no longer the verbatim in the system. Suggestions were not updated for the coding request.",
"id": "V0V000000003003",
"dictionary_release": "MedDRA_25_1",
"verbatim": "Severe back pain"
}
}
Notes
- The action is used to set external coding suggestions to specific Medical Coding Requests.
- The action will DELETE existing suggestions (for a request that has them currently), and replace with the new set posted (no upsert)
- To unset a request that has suggestions to have none, simply use
"external_suggestions": []. (empty array of suggestions) - Each suggestion (of request) is validated against a match of dictionary version (current on the Med Coding Request) and vendor/company name.
- Optionally include verbatim / route / indication values with the post of suggestions. This will check that the values have not changed recently, i.e. perhaps the suggestions no longer apply.
- Each Study must be activated in it’s Study settings to use external suggestions. The company/vendor on the post then also must match the API Name as set up in the Vault for that company/vendor.
- WARNING: For WHODrug coding, whether the ATC1 (or other levels) is required depends on that Study's setting in the Vault for medical coding.
- Suggestions posted to one coding request is limited to 5.
- The limit for actions in one API post is 100. (coding requests)
- Use Retrieve Coding Requests, including suggestions in the response to observe current state of requests to their suggestions. The parameter/value of
has_suggestions=falsewill filter to requests needing suggestions.
Endpoint
POST /api/{version}/app/cdm/coder/actions/setcodingsuggestions
Required Permissions
The following permissions are required to use the Open Coding Queries API:
- API Access
- View Casebook
- Manage Coding
The role CDMS API Read Write grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Array | Description |
|---|---|---|
study_name |
Name of the Study | |
external_definition_name |
The API Name of the external suggestions definition in the Vault. This is setup once, then must also be applied to the Studies that will use the external suggestions concept. | |
id |
requests |
The id value for the Medical Coding Request. These values can be found in the responses from Retrieve Coding Requests |
dictionary_release |
requests |
This value must match the current dictionary version (name__v value) of that coding Form in the Study. The current value can be found both at Retrieve Coding Definitions and with each response entry returned in Retrieve Coding Requests. If passed in the attempt, and it does not match the current value in EDC, the attempt on the coding request is rejected. |
verbatim |
requests |
Optional When passed with the attempt, the current value of the value is checked in the Study. This is helpful in ensuring the value used in the external coding algorithm is still the same in the Study, at the time of posting the suggestions. Later changes to this in the system will appropriately remove suggestions since they no longer apply. If the value is not passed, the extra check is not performed. |
indication |
requests |
Optional (WHODrug only) When passed with the attempt, the current value of the value is checked in the Study. This is helpful in ensuring the value used in the external coding algorithm is still the same in the Study, at the time of posting the suggestions. Later changes to this in the system will appropriately remove suggestions since they no longer apply. If the value is not passed, the extra check is not performed. WARNING: A Study must be configured for the coding Form to use this property. |
route |
requests |
Optional (WHODrug only) When passed with the attempt, the current value of the value is checked in the Study. This is helpful in ensuring the value used in the external coding algorithm is still the same in the Study, at the time of posting the suggestions. Later changes to this in the system will appropriately remove suggestions since they no longer apply. If the value is not passed, the extra check is not performed. WARNING: A Study must be configured for the coding Form to use this property. |
external_id |
../external_suggestions |
Optional Use this field to set a value meaningful to the remote system / algorithm, e.g. an ID from a database storing these suggestion combinations. |
relevance_score |
../external_suggestions |
This is the value for the suggestion that ranks it amongst other suggestions of the same request. The higher the number, the better the ranking, i.e. appearing at the top in the user interface. For example, suggestions with relevance score of 10, 70, 40, 95, 20 in the post of five suggestions would be ordering in the Coder user interface (reading down) in order 95,70,40,20,10 |
external_version |
../external_suggestions |
Optional Use this field to set a value meaningful to the remote system / algorithm, e.g. its version when the suggestion was generated. |
soc_code |
../external_suggestions |
(MedDRA only) The soc_code value for the suggestion |
soc |
../external_suggestions |
(MedDRA only) The soc value for the suggestion |
hlgt_code |
../external_suggestions |
(MedDRA only) The hlgt_code value for the suggestion |
hlgt |
../external_suggestions |
(MedDRA only) The hlgt value for the suggestion |
hlt_code |
../external_suggestions |
(MedDRA only) The hlt_code value for the suggestion |
hlt |
../external_suggestions |
(MedDRA only) The hlt value for the suggestion |
pt_code |
../external_suggestions |
(MedDRA only) The pt_code value for the suggestion |
pt |
../external_suggestions |
(MedDRA only) The pt value for the suggestion |
llt_code |
../external_suggestions |
(MedDRA only) The llt_code value for the suggestion |
llt |
../external_suggestions |
(MedDRA only) The llt value for the suggestion |
primary_path |
../external_suggestions |
Optional (MedDRA only) The primary path value for the suggestion - only values of Y (Yes) or N (No) allowed. |
drug_code |
../external_suggestions |
(WHODrug only) The drug_code value for the suggestion |
drug_name |
../external_suggestions |
(WHODrug only) The drug_name value for the suggestion |
preferred_code |
../external_suggestions |
(WHODrug only) The preferred_code value for the suggestion |
preferred_name |
../external_suggestions |
(WHODrug only) The preferred_name value for the suggestion |
atc1_code |
../external_suggestions |
Optional (!) (WHODrug only) WARNING: Can be Required, depending on Study configuration. The atc1_code value for the suggestion |
atc1 |
../external_suggestions |
Optional (!) (WHODrug only) WARNING: Can be Required, depending on Study configuration. The atc1 value for the suggestion |
atc2_code |
../external_suggestions |
Optional (WHODrug only) The atc2_code value for the suggestion |
atc2 |
../external_suggestions |
Optional (WHODrug only) The atc2 value for the suggestion |
atc3_code |
../external_suggestions |
Optional (WHODrug only) The atc3_code value for the suggestion |
atc3 |
../external_suggestions |
Optional (WHODrug only) The atc3 value for the suggestion |
atc4_code |
../external_suggestions |
Optional (WHODrug only) The atc4_code value for the suggestion |
atc4 |
../external_suggestions |
Optional (WHODrug only) The atc4 value for the suggestion |
Jobs
Job Summary / Types
For Clinical Data, a Job (also known as Study Job) processes against a Study, either updating data, or generating information about the Study. In the current release, we only support data extract related jobs in the API.
The following job types are currently supported:
| Name | API Name | Description |
|---|---|---|
| Study Data Extract (SDE) | study_data_extract__v |
Generates ZIP of CSV files (with/without SAS and SAS XPT files). The file includes clinical datasets (Form data), system/operational datasets, custom objects involved with the Study, and/or Study definitions. This export is the primary and most comprehensive full export of Study data. It can be run in subset for faster execution, e.g., just Adverse Events, SYS_Q (queries), and so forth. Learn more about the content of the SDE can be found at Clinical Data Help |
| Subject Progress Listing | subject_progress_listing__v |
Generates CSV of metrics at Subject level. This summary is not Study design specific, instead primarily operational data, statuses, row per Subject |
| Subject Progress Listing (Versioned) | subject_progress_versioned_extract__v |
(Requires Veeva enablement for your Vault) Same/similar content as subject_progress_listing__v, but with a version required. Similar to the SDE in that the structure of the output won't change for a version consistently supplied to start (or schedule) the job. Additionally, this job type can also be forwarded to a Veeva FTP site on completion. |
| Event Progress Listing | event_progress_listing__v |
Generates CSV of metrics at Event level. This summary is not Study design specific, although is a 'row per event' of the Study design. Each row includes operational / status information about the Event. |
| Event Progress Listing (Versioned) | event_progress_versioned_extract__v |
(Requires Veeva enablement for your Vault) Same/similar content as event_progress_listing__v, but with a version required. Similar to the SDE in that the structure of the output won't change for a version consistently supplied to start (or schedule) the job. Additionally, this job type can also be forwarded to a Veeva FTP site on completion. |
| Form Progress Listing | form_progress_listing__v |
Generates CSV of metrics at Form level. This summary is not Study design specific, although is a 'row per Form' of the Study design. Each row includes operational / status information about the Form. |
| Form Progress Listing (Versioned) | form_progress_versioned_extract__v |
(Requires Veeva enablement for your Vault) Same/similar content as form_progress_listing__v, but with a version required. Similar to the SDE in that the structure of the output won't change for a version consistently supplied to start (or schedule) the job. Additionally, this job type can also be forwarded to a Veeva FTP site on completion. |
| Query Detail Listing | query_detail_listing__v |
Generates CSV of metrics at Query level. This summary is not Study design specific, although each row will indicate the location (of Study design) of the Query. |
| Query Detail Listing (Versioned) | query_detail_versioned_extract__v |
(Requires Veeva enablement for your Vault) Same/similar content as query_detail_listing__v, but with a version required. Similar to the SDE in that the structure of the output won't change for a version consistently supplied to start (or schedule) the job. Additionally, this job type can also be forwarded to a Veeva FTP site on completion. |
| Core Listing | core_listing__v |
Predecessor to the SDE. Generates ZIP of CSVs for each Form, but without Study definition. It can be run on subset(s) of Forms and/or Sites. (Format different from the SDE) |
| Data and Definition Export (Deprecated) | data_and_definition_export__v |
Predecessor to the SDE, also once known as the JReview job type. Generates a ZIP file of CSVs representing collected data in your Study, as well as definitions. (Format different from the SDE) |
| Audit Trail by Study | audit_trail_export__v |
Job that produces audit trail reporting, with specified start to end date ranges and/or specific users. The result of the job is a zip file containing CSVs, by Subject. The range of start to end can be no more than 30 days when starting this job from the API. Wider ranges are started from the EDC Tools user interface. |
| Audit Trail by Site | audit_trail_export_by_site__v |
Job that produces audit trail reporting, with specified start to end date ranges, specific users, and/or specific sites. The result of the job is a zip file containing CSVs, by Subject. The range of start to end can be no more than 30 days when starting this job from the API. Wider ranges are started from the EDC Tools user interface. |
| Audit Trail by Subject | audit_trail_export_by_subject__v |
Job that produces audit trail reporting, with specified start to end date ranges, specific users, and/or specific Subjects. The result of the job is a zip file containing CSVs, by Subject. The range of start to end can be no more than 30 days when starting this job from the API. Wider ranges are started from the EDC Tools user interface. |
| Data Change Report | data_change_report__v |
Job that produces changes to Items, with specified start to end date ranges, Forms (or all), and with/without restricted data. Optionally, send the resulting results to a Vault FTP folder. The range of start to end can be no more than 90 days when starting this job from the API, and the job can be configured with just 'Last X Days' (vs. specific dates). Wider ranges are started from the EDC Tools user interface. |
| Casebook Design Export | casebook_design_export__v |
Job that generates a JSON file of the Study design. Included in the content are the schedule of the Study, all Form designs of the schedule, codelists, unit definition, coding definitions, and Study settings. Each job run (file) is one to one with a specific casebook version of the Study design. |
| Safety Follow-Up Scan | safety_integrations_follow_up_scan__v |
For Studies with the EDC Safety Integrations module enabled, this job will start a job that scans for any appropriate follow-up sends to a safety system. The job can be run to detect recent changes (since last job run) OR in full (scanning every safety case). If a scheduled job is in place for the Study, this is largely unnecessary. Instead, it's purpose is for cases where the start of the scanning is to be driven by conditions outside Clinical Data. |
For cases where a recurring job already runs, Retrieve Study Jobs can be used to detect recently finished jobs, across multiple Studies. In this way, a single integration API job can easily detect new Studies deployed to a Vault and retrieve now executing data export files.
If using the API to start a Job, you can monitor the status of a Job using Retrieve Job Status or in the UI by accessing Tools > EDC Tools > Job History.
To download the output files for a Job, use Retrieve Job Output File or download from Tools > EDC Tools > Job History.
Learn more about managing jobs in Clinical Data Help.
Retrieve Study Jobs
Request - Single Study, SDE type, default date filters, no status filter (7 days ago to now, assuming run on July 1st, 2022)
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs?study_name=ABCP-2022-01_DEV1&&job_type=study_data_extract__v' \
-H 'Authorization: {SESSION_ID}'
Response - Single Study, SDE type, default date filters, no status filter (7 days ago to now, assuming run on July 1st, 2022)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"created_start": "2022-06-01T12:00:00Z",
"created_end": null,
"jobs": [
{
"job_id": 324003,
"job_type": "study_data_extract__v",
"study_name": "ABCP-2022-01_DEV1",
"status": "completed__v",
"created_by": "John Smith",
"created_date": "2022-06-02T12:14:42Z",
"last_modified_date": "2022-06-02T12:20:42Z"
},
{
"job_id": 324002,
"job_type": "study_data_extract__v",
"study_name": "ABCP-2022-01_DEV1",
"status": "errors__v",
"created_by": "John Smith",
"created_date": "2022-06-01T08:14:42Z",
"last_modified_date": "2022-06-01T08:02:42Z"
}
]
}
Request - Multiple Studies, SDE type, specific range of job created dates, no status filter
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs?job_type=study_data_extract__vcreated_start=2022-05-20T12:00:00Z&created_end=2022-06-01T12:00:00Z' \
-H 'Authorization: {SESSION_ID}'
Response - Multiple Studies, SDE type, specific range of job created dates, no status filter
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 5,
"total": 5
},
"created_start": "2022-05-20T12:00:00Z",
"created_end": "2022-06-01T12:00:00Z",
"jobs": [
{
"job_id": 324002,
"job_type": "study_data_extract__v",
"study_name": "ABCP-2022-01_DEV1",
"status": "errors__v",
"created_by": "John Smith",
"created_date": "2022-06-01T08:14:42Z",
"last_modified_date": "2022-06-01T08:02:42Z"
},
{
"job_id": 323991,
"job_type": "study_data_extract__v",
"study_name": "ABCP-2022-01_DEV1",
"status": "completed__v",
"created_by": "John Smith",
"created_date": "2022-05-31T09:15:42Z",
"last_modified_date": "2022-05-31T17:02:42Z"
},
{
"job_id": 323923,
"job_type": "study_data_extract__v",
"study_name": "ABCP-XYZ-REG-01_DEV1",
"status": "completed__v",
"created_by": "John Smith",
"created_date": "2022-05-29T13:31:42Z",
"last_modified_date": "2022-05-29T13:33:42Z"
},
{
"job_id": 323912,
"job_type": "study_data_extract__v",
"study_name": "ABCP-2022-01_DEV1",
"status": "completed__v",
"created_by": "John Smith",
"created_date": "2022-05-25T07:54:42Z",
"last_modified_date": "2022-05-25T08:02:41Z"
},
{
"job_id": 323898,
"job_type": "study_data_extract__v",
"study_name": "ABCP-XYZ-REG-01_DEV1",
"status": "completed__v",
"created_by": "John Smith",
"created_date": "2022-05-21T08:14:42Z",
"last_modified_date": "2022-05-21T08:16:42Z"
}
]
}
Notes
- Used to retrieve recent Study Jobs, across one or more Studies in one request.
- The primary use case is for API automation of retrieving export files, i.e., already setup and scheduled.
- To view a complete list of all job runs, you must also have the Restricted Data permission. Jobs associated with restricted data access are masked from users without it.
Endpoint
GET /api/{version}/app/cdm/jobs
Required Permissions
The following permissions are required to use the Retrieve Study Jobs API:
- API Access
- Manage Jobs
The CDMS API Read Write and CDMS API Read Only roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Optional Name of the Study. This API supports retrieve across multiple Studies in one request |
job_type |
The specific type of Job to retrieve. Only one type can be used per request. Valid job types can be found at Job Summary / Types |
created_start |
Optional Retrieve only Jobs after this datetime of creation. Use the format "yyyy-MM-ddTHH:mm:ssZ". When omitted, 'Now' - 7 days is used. The difference between created_start and created_end - provided or not - can be no more than 30 days. |
created_end |
Optional Retrieve only Jobs before this datetime of creation. Use the format "yyyy-MM-ddTHH:mm:ssZ". When omitted, 'Now' is used. The difference between created_start and created_end - provided or not - can be no more than 30 days. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of Study Jobs (as JSON array entries) with the following information:
| Array | Name | Description |
|---|---|---|
created_start |
If passed in the request, re-iterated, otherwise the default value. | |
created_end |
If passed in the request, re-iterated, otherwise the default value. | |
jobs |
job_id |
The ID of the Vault Job tied to the Study Job. Use this value in Retrieve Job Output File to retrieve the file content of the job |
jobs |
job_type |
The type of the Job (re-iterate of the request value) |
jobs |
study_name |
Name of the Study of the Job |
jobs |
status |
The current status of the job, e.g. completed__v, errors__v, in_progress__v |
jobs |
created_by |
The user who started the Job |
jobs |
created_date |
Format yyyy-MM-ddThh:ss:mmZ, UTC date time |
jobs |
last_modified_date |
Format yyyy-MM-ddThh:ss:mmZ, UTC date time |
Start Job by Type
Each job requires different body parameters. Each section below uses the same endpoint (Start Job), but lists the parameters and other details specific to that section's job type.
Study Data Extract (SDE)
Request - Start SDE
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "study_data_extract__v",
"include_restricted_data": true,
"include_study_design": true,
"version": "v24.1",
"export_file_types": ["CSV", "SAS"],
"file_name": "ABCP-2022-01_DEV1_Study_Data_Extract",
"use_external_ids": false,
"include_formilb": true,
"split_datetime": false,
"exclude_blank_forms": false,
"all_clinical_datasets": true,
"all_system_datasets": true,
"include_custom_objects": false
}
}'
Response - Start SDE
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "study_data_extract__v",
"job_id": 522518,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T18:18:54Z",
"include_restricted_data": true,
"include_study_design": true,
"version": "v24.1",
"export_file_types": ["CSV", "SAS"],
"file_name": "ABCP-2022-01_DEV1_Study_Data_Extract",
"external_connections": null,
"use_external_ids": false,
"include_formilb": true,
"split_datetime": true,
"exclude_blank_forms": false,
"include_rand_treatment": false,
"all_clinical_datasets": true,
"clinical_datasets": null,
"all_system_datasets": true,
"system_datasets": null,
"include_custom_objects": false,
"custom_objects": null,
"boolean_formatting": "y_n"
}
}
Notes
The Study Data Extract (SDE) job allows the extract of Study execution data from Vault, in CSV (UTF-8), and optional SAS/SAS XPT format. The job's output includes Study datasets (AE, CM, etc.) as configured in the Study design, plus the following system datasets:
- SYS_SITE: Lists all Sites.
- SYS_SUB: Lists all Subjects.
- SYS_EVT: Lists all Events (visits) with the Event Date and Status.
- SYS_FORM: Lists all Forms, including any Forms marked as intentionally left blank, with the Review Status, Freeze Status, Lock Status, and some additional metrics.
- SYS_Q: Lists all Queries, along with some Query metrics. Only the first Query message is listed in this dataset. The full list of Query messages (first message, answers, etc.) is included in the SYS_QT dataset.
- SYS_QT: Lists all Query Messages.
- SYS_ILB: Lists all Items that are marked as intentionally left blank.
- SYS_LINKS: Lists all Forms that are part of Form to Form linking (or Form to Item). Each link is a pair of records in the dataset.
- SYS_ASM: Lists all Assessments that exist in the Study, if Assessments are configured in the Study design.
- SYS_ASMR: Lists all Questions and Answers for all Assessments, if Assessments are configured in the Study design.
- SYS_PD: Lists all Protocol Deviations, if this feature is enabled in the Study.
- SYS_RAND: Lists randomization details, if Randomization is enabled for the Study.
- SYS_LABRANGES: Lists Normal Ranges, if Local Labs is enabled for the Study.
- SYS_LABLOC: Lists Lab Locations, if Local Labs is enabled for the Study.
- SYS_ANALYTES: Lists Analytes, if Local Labs is enabled for the Study.
See Clinical Data Help for a detailed list of all columns in the Study Data Extract output.
Custom objects - if involved in the Study design - are also included as datasets. Definition file(s) of Study design are also included (differs by SDE version):
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Study Data Extract Job API:
- API Access
- Manage Jobs
- View Casebook
The CDMS API Read Write role grants the API Access, Manage Jobs, and View Casebook permissions.
Additional permissions:
- Restricted Data Access: This permission is also required when including restricted data in the job output.
- View Randomization Enrollment: This permission is also required when including randomization treatment information in the job output of an unmasked study.
- View Unmasked Data & View Randomization Enrollment: This permission is also required when including randomization treatment information in the job output of a masked study.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of study_data_extract__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
include_study_design |
Optional. true/false, false when omitted. Whether the run should include additional CSVs about the Study design. |
version |
The SDE version (not API version) to use for the export run. Formats/options are different by SDE version. Values are of format vX.Y, e.g. v21.2, v21.3, v22.1, v22.2, v22.3, v23.1, v23.2, v23.3, v24.1 etc. |
export_file_types |
The type(s) of files of export. The is a JSON array of strings, with valid values of CSV, SAS, and/or XPT, in any order. Previous to SDE version v23.2, this was instead just export_file_type with different choices. If an older SDE version is desired for the start of the job, refer to that API release documentation for valid values using export_file_type. |
file_name |
Optional Filename to use for the resulting ZIP file (up to 200 characters). If omitted, Vault names the file "{Study_Name}_Study_Data_Extract_{DATETIME}_{TIMEZONE}.zip", with a 'SAS_' prefix when the export includes SAS/SAS XPT files. |
external_connections |
Optional. JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
use_external_ids |
Optional true/false, false when omitted. Whether to use the Form / Item design External IDs for the naming of datasets and dataset columns. The default is to use Form / Item design name. |
include_formilb |
Optional true/false, false when omitted. Whether the export run should include clinical dataset rows for Forms in status of Intentionally Left Blank (ILB), or not. |
split_datetime |
Optional true/false, false when omitted. Whether all datetime Item fields should be split into separate (additional) dataset columns for date only / time only |
exclude_blank_forms |
Optional true/false, false when omitted. Whether to exclude data from Forms in status blank__v, or not |
include_rand_treatment |
Optional true/false, false when omitted. Only applicable if the Study is using the Randomization module within EDC, and for those with unmasked permissions in the module. The result (with parameter true) will be treatment values within the SYS_RAND dataset. |
all_clinical_datasets |
true/false - whether all clinical datasets (e.g. Forms/CRFs) should be included or not. When using false, then specific ones must be listed in the clinical_datasets parameter |
clinical_datasets |
Optional JSON array of names of specific clinical datasets to include. The names are those of the Form Study design names.
|
all_system_datasets |
true/false - whether all SYS_* datasets should be included or not. When using false, then specific ones must be listed in the system_datasets parameter |
system_datasets |
Optional JSON array of names of system datasets to include. Only those system datasets that are applicable for the Study's design settings are valid.
|
include_custom_objects |
true/false, false when omitted. |
custom_objects |
Optional Must provide when include_custom_objects = true. JSON array of names of custom object configuration to include in the export. These values are those found in sde_customobject_config__v.name__v for the Study. (usually the same as the object name itself). These are configured via Admin -> Business Admin only at current release. Example, assuming just two custom objects (document_tracking_c / cra_visit_trackingc) with name_v values of CustomObj1_Docs and CustomObj2_VisitTracking: "custom_objects": [ "CustomObj1_Docs", "CustomObj2_VisitTracking" ] |
boolean_formatting |
Optional. The format to use for boolean/checkbox values, y_n when omitted. Possible values are y_n, y_n_empty, t_f, t_f_empty, 1_0, 1_empty, 1_0_empty, true_false, true_false_empty, yes_no, yes_no_empty |
item_display_label |
Optional. The format to use for SAS labels. Possible values are label and short_label. When omitted, the default is label. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: study_data_extract__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
include_study_design |
Parameter provided, or the default if omitted. |
version |
Parameter provided in the request |
export_file_type |
Parameter provided, or the default if omitted. |
file_name |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
use_external_ids |
Parameter provided, or the default if omitted. |
include_formilb |
Parameter provided, or the default if omitted. |
split_datetime |
Parameter provided, or the default if omitted. |
exclude_blank_forms |
Parameter provided, or the default if omitted. |
include_rand_treatment |
Parameter provided, or the default if omitted. |
all_clinical_datasets |
Parameter provided, or the default if omitted. |
clinical_datasets |
Parameter provided, or null if omitted |
all_system_datasets |
Parameter provided, or the default if omitted. |
system_datasets |
Parameter provided, or null if omitted |
include_custom_objects |
Parameter provided, or the default if omitted. |
custom_objects |
Parameter provided, or null if omitted |
boolean_formatting |
Parameter provided, or the default if omitted (y_n) |
Subject Progress Listing
Request - Start Subject Progress Listing
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "subject_progress_listing__v",
"include_restricted_data": true
}
}'
Response - Start Subject Progress Listing
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "subject_progress_listing__v",
"job_id": 525007,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:09:54Z",
"include_restricted_data": true
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Subject Progress Listing Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of subject_progress_listing__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: subject_progress_listing__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
Subject Progress Listing (Versioned)
Request - Start Subject Progress Listing (Versioned)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "subject_progress_versioned_extract__v",
"version": "v24.1",
"include_restricted_data": true,
"external_connections": ["My_FTP_Drop"]
}
}'
Response - Start Subject Progress Listing (Versioned)
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "subject_progress_versioned_extract__v",
"job_id": 525007,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:09:54Z",
"version": "v24.1",
"include_restricted_data": true,
"external_connections": ["My_FTP_Drop"]
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Subject Progress Listing Job (Versioned) API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of subject_progress_versioned_extract__v in this case, i.e., for start of this type of job. |
version |
The version of the output to generate. Example - v24.1. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
external_connections |
Optional JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: subject_progress_versioned_extract__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
version |
Parameter provided in the request. |
include_restricted_data |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
Event Progress Listing
Request - Start Event Progress Listing
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "event_progress_listing__v",
"include_restricted_data": true,
"include_review_data": true
}
}'
Response - Start Event Progress Listing
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "event_progress_listing__v",
"job_id": 525008,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:11:35Z",
"include_restricted_data": true,
"include_review_data": true
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Event Progress Listing Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of event_progress_listing__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
include_review_data |
Optional. true/false, false when omitted. Whether the run of data should include data about review activity (SDV / DMR). The API caller must have the permission for view of this information when using true on this option. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: event_progress_listing__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
include_review_data |
Parameter provided, or the default if omitted. |
Event Progress Listing (Versioned)
Request - Start Event Progress Listing (Versioned)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "event_progress_versioned_extract__v",
"version": "v24.1",
"include_restricted_data": true,
"include_review_data": true,
"external_connections": ["My_FTP_Drop"]
}
}'
Response - Start Event Progress Listing (Versioned)
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "event_progress_versioned_extract__v",
"job_id": 525008,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:11:35Z",
"version": "v24.1",
"include_restricted_data": true,
"include_review_data": true,
"external_connections": ["My_FTP_Drop"]
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Event Progress Listing Job (Versioned) API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of event_progress_versioned_extract__v in this case, i.e., for start of this type of job. |
version |
The version of the output to generate. Example - v24.1. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
include_review_data |
Optional. true/false, false when omitted. Whether the run of data should include data about review activity (SDV / DMR). The API caller must have the permission for view of this information when using true on this option. |
external_connections |
Optional JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: event_progress_versioned_extract__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
version |
Parameter provided in the request. |
include_restricted_data |
Parameter provided, or the default if omitted. |
include_review_data |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
Form Progress Listing
Request - Start Form Progress Listing
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "form_progress_listing__v",
"include_restricted_data": true,
"include_item_counts": true
}
}'
Response - Start Form Progress Listing
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "form_progress_listing__v",
"job_id": 525105,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:14:29Z",
"include_restricted_data": true,
"include_item_counts": true
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Form Progress Listing Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of form_progress_listing__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
include_item_counts |
Optional. true/false, false when omitted. Whether to include a count of existing Items in each row, i.e., Items on that Form. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: form_progress_listing__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
include_item_counts |
Parameter provided, or the default if omitted. |
Form Progress Listing (Versioned)
Request - Start Form Progress Listing (Versioned)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "form_progress_versioned_extract__v",
"version": "v24.1",
"include_restricted_data": true,
"include_item_counts": true,
"external_connections": ["My_FTP_Drop"]
}
}'
Response - Start Form Progress Listing (Versioned)
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "form_progress_versioned_extract__v",
"job_id": 525105,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:14:29Z",
"version": "v24.1",
"include_restricted_data": true,
"include_item_counts": true,
"external_connections": ["My_FTP_Drop"]
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Form Progress Listing (Versioned) Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of form_progress_versioned_extract__v in this case, i.e., for start of this type of job. |
version |
The version of the output to generate. Example - v24.1. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
include_item_counts |
Optional. true/false, false when omitted. Whether to include a count of existing Items in each row, i.e., Items on that Form. |
external_connections |
Optional JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: form_progress_versioned_extract__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
version |
Parameter provided in the request. |
include_restricted_data |
Parameter provided, or the default if omitted. |
include_item_counts |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
Query Detail Listing
Request - Start Query Detail Listing
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "query_detail_listing__v",
"include_restricted_data": true
}
}'
Response - Start Query Detail Listing
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "query_detail_listing__v",
"job_id": 525006,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:09:06Z",
"include_restricted_data": true
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Query Detail Listing Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of query_detail_listing__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: query_detail_listing__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
Query Detail Listing (Versioned)
Request - Start Query Detail Listing (Versioned)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "query_detail_versioned_extract__v",
"version": "v24.1",
"include_restricted_data": true,
"external_connections": ["My_FTP_Drop"]
}
}'
Response - Start Query Detail Listing (Versioned)
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "query_detail_versioned_extract__v",
"job_id": 525006,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-12-23T00:09:06Z",
"version": "v24.1",
"include_restricted_data": true,
"external_connections": ["My_FTP_Drop"]
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Query Detail Listing (Versioned) Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of query_detail_versioned_extract__v in this case, i.e., for start of this type of job. |
version |
The version of the output to generate. Example - v24.1. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
external_connections |
Optional JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: query_detail_versioned_extract__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
version |
Parameter provided in the request. |
include_restricted_data |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
Core Listings
Request - Start Core Listings
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-D '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "core_listing__v",
"all_sites": true,
"sites": [],
"all_forms": true,
"forms": []
}
}'
Response - Start Core Listings
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "core_listing__v",
"job_id": 522516,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T17:45:52Z",
"all_sites": true,
"sites": null,
"all_forms": true,
"forms": null
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Core Listing Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of core_listing__v in this case, i.e., for start of this type of job. |
all_sites |
Optional true/false, false when omitted, i.e., specific Sites must be provided in sites. |
sites |
Optional Must provide when all_sites = false. JSON array of names of specific Sites to retrieve data for. Example, assuming just two of the Sites: "sites": [ "101", "102" ] |
all_forms |
Optional true/false, false when omitted, i.e., specific Form design names must be provided in forms. |
forms |
Optional Must provide when all_forms = false. JSON array of design names of Forms to include. Example, assuming just two of the Forms: "forms": [ "DM", "AE" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: core_listing__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
all_sites |
Parameter provided, or the default if omitted. |
sites |
Parameter provided, or null if omitted |
all_forms |
Parameter provided, or the default if omitted. |
forms |
Parameter provided, or null if omitted |
Data and Definition Export (Deprecated)
Request - Start DDE
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "data_and_definition_export__v",
"include_restricted_data": true
}
}'
Response - Start DDE
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "data_and_definition_export__v",
"job_id": 522417,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T18:10:01Z",
"include_restricted_data": true
}
}
This job is retired. Contact your Veeva Services Representative to obtain explicit usage permissions. The Study Data Extract (SDE) job has been enhanced to replace the functionality previously provided by the Data and Definition Export job, including access to the study's definition files.
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Data and Definition Export Job API:
- API Access
- Manage Jobs OR Manage Data and Definition Export
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
To include restricted data in the job output, you must also have the Restricted Data Access permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of data_and_definition_export__v in this case, i.e., for start of this type of job. |
include_restricted_data |
Optional. true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: data_and_definition_export__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
include_restricted_data |
Parameter provided, or the default if omitted. |
Audit Trail by Study
Request - Start Audit Trail by Study
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "audit_trail_export__v",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03"
}
}'
Response - Start Audit Trail by Study
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "audit_trail_export__v",
"job_id": 522417,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T18:10:01Z",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03",
"specific_users": null
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Audit Trail by Study API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of audit_trail_export__v in this case, i.e., for start of this type of job. |
date_range_start |
YYYY-MM-DD format, the date from which to retrieve audit trail. The start to end range can be no more than 30 days. |
date_range_end |
Optional. YYYY-MM-DD format, the date retrieve audit trail through / up to. The start to end range can be no more than 30 days. When omitted, today is assumed. |
specific_users |
Optional. Specific users to obtain audit trail about, as JSON array of users names. For example - "specific_users": ["joe.smith@abcpharma.com", "mary.jones@abcpharma.com"] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: audit_trail_export__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
date_range_start |
Parameter provided. |
date_range_end |
Parameter provided, or the default if omitted. |
specific_users |
Parameter provided, or null if omitted. |
Audit Trail by Site
Request - Start Audit Trail by Site
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "audit_trail_export_by_site__v",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03",
"specific_sites": ["101", "102"]
}
}'
Response - Start Audit Trail by Site
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "audit_trail_export_by_site__v",
"job_id": 522417,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T18:10:01Z",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03",
"specific_users": null,
"specific_sites": ["101", "102"]
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Audit Trail by Site API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of audit_trail_export_by_site__v in this case, i.e., for start of this type of job. |
date_range_start |
YYYY-MM-DD format, the date from which to retrieve audit trail. The start to end range can be no more than 30 days. |
date_range_end |
Optional. YYYY-MM-DD format, the date retrieve audit trail through / up to. The start to end range can be no more than 30 days. When omitted, today is assumed. |
specific_sites |
Specific sites to obtain audit trail about, as JSON array of Site name/number. For example - "specific_sites": ["101", "201"] |
specific_users |
Optional. Specific users to obtain audit trail about, as JSON array of users names. For example - "specific_users": ["joe.smith@abcpharma.com", "mary.jones@abcpharma.com"] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: audit_trail_export_by_site__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
date_range_start |
Parameter provided. |
date_range_end |
Parameter provided, or the default if omitted. |
specific_sites |
Parameter provided. |
specific_users |
Parameter provided, or null if omitted. |
Audit Trail by Subject
Request - Start Audit Trail by Subject
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "audit_trail_export_by_subject__v",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03",
"specific_subjects": ["101-001", "101-002"]
}
}'
Response - Start Audit Trail by Subject
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "audit_trail_export_by_subject__v",
"job_id": 522417,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-05-05T18:10:01Z",
"date_range_start": "2023-05-01",
"date_range_end": "2023-05-03",
"specific_users": null,
"specific_subjects": ["101-001", "101-002"],
"deleted_subjects": false
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Start Audit Trail by Site API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of audit_trail_export_by_subject__v in this case, i.e., for start of this type of job. |
date_range_start |
YYYY-MM-DD format, the date from which to retrieve audit trail. The start to end range can be no more than 30 days. |
date_range_end |
Optional. YYYY-MM-DD format, the date retrieve audit trail through / up to. The start to end range can be no more than 30 days. When omitted, today is assumed. |
specific_subjects |
Specific Subjects to obtain audit trail about, as JSON array of Subject name/number. For example - "specific_subjects": ["101-001", "101-002"] |
specific_users |
Optional. Specific users to obtain audit trail about, as JSON array of users names. For example - "specific_users": ["joe.smith@abcpharma.com", "mary.jones@abcpharma.com"] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: audit_trail_export_by_subject__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
date_range_start |
Parameter provided. |
date_range_end |
Parameter provided, or the default if omitted. |
specific_subjects |
Parameter provided. |
specific_users |
Parameter provided, or null if omitted. |
deleted_subjects |
Always false for clarity, as it cannot be specified with the start of the job via API (at this time). For audit trail on deleted Subjects, use instead the EDC Tools user interface. |
Data Change Report
Request - Start Data Change report - (open ended date range, today is July 14th, all Forms, no restricted data)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "data_change_report__v",
"export_file_type": "CSV",
"date_range_start": "2023-07-10",
"include_restricted_data": false,
"all_forms": true
}
}'
Response - Data Change Report
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "data_change_report__v",
"job_id": 307483,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-06-27T20:27:26Z",
"export_file_type": "CSV",
"date_range_start": "2023-07-10",
"date_range_end": "2023-07-14",
"days_previous": null,
"include_restricted_data": false,
"all_forms": true,
"forms": null,
"external_connections": null
}
}
Request - Start Data Change report - (restricted data included, using days previous, specific Forms, Excel, and FTP)
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "data_change_report__v",
"export_file_type": "Excel",
"date_range_start": null,
"date_range_end": null,
"days_previous": 5,
"include_restricted_data": true,
"all_forms": false,
"forms": ["AE", "CM", "DM"],
"external_connections": ["MyFTPLocation"]
}
}'
Response - Data Change Report
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "data_change_report__v",
"job_id": 307483,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-06-27T20:27:26Z",
"export_file_type": "Excel",
"date_range_start": null,
"date_range_end": null,
"days_previous": 5,
"include_restricted_data": true,
"all_forms": false,
"forms": ["AE", "CM", "DM"],
"external_connections": ["MyFTPLocation"]
}
}
The Data Change Report is referred to as the Data Change Extract in the EDC Tools user interface. The report includes changes to Items and Event Dates during a specific date range.
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Data Change Report API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of data_change_report__v in this case, i.e., for start of this type of job. |
export_file_type |
Values of CSV or Excel only. |
date_range_start |
Optional (Start to end, or days previous is required, but not both) YYYY-MM-DD format, the date from which to retrieve item data changes. The start to end range can be no more than 90 days. |
date_range_end |
Optional (Start to end, or days previous is required, but not both) YYYY-MM-DD format, the date retrieve item data changes through / up to. The start to end range can be no more than 90 days. When omitted, today is assumed. |
days_previous |
Optional (Start to end, or days previous is required, but not both) Whole numbers from 1 to 90 only. |
include_restricted_data |
Optional true/false, false when omitted. Whether the run of data should include data from restricted Forms (or not). The API caller must have the permission for the restricted data when using true on this option. |
all_forms |
Optional true/false, true when omitted. Whether to filter to a specific list of Forms, by design name, or not. |
forms |
Optional (Must provide when all_forms = false) JSON array of names of Form design names to include. |
external_connections |
Optional JSON array of names of FTP connection names to also push a copy of the zip file to. These connections are setup with the Study, in the Tools -> EDC Tools -> FTP area. Example, assuming send to two FTP locations: "external_connections": [ "My_FTP_Connection1", "CRO_Connector" ] |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: data_change_report__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
export_file_type |
Parameter provided. |
date_range_start |
Parameter provided, or the default if omitted. |
date_range_end |
Parameter provided, or the default if omitted. |
days_previous |
Parameter provided, or the default if omitted. |
include_restricted_data |
Parameter provided, or the default if omitted. |
all_forms |
Parameter provided, or the default if omitted. |
forms |
Parameter provided, or the default if omitted. |
external_connections |
Parameter provided, or the default if omitted. |
Casebook Design Export (CDE)
Request - Start CDE
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "casebook_design_export__v",
"casebook_version": 1
}
}'
Response - CDE
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "casebook_design_export__v",
"job_id": 307483,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-06-27T20:27:26Z",
"casebook_version": 1
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Casebook Design Export Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of casebook_design_export__v in this case, i.e., for start of this type of job. |
casebook_version |
Optional When not provided, the latest casebook version is used for the output generated. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: casebook_design_export__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
casebook_version |
Parameter provided, or the default if omitted. |
Safety Follow-Up Scan
Request - Safety Follow-Up Scan
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/start_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '{
"study_name": "ABCP-2022-01_DEV1",
"request": {
"job_type": "safety_integrations_follow_up_scan__v",
"full_scan": true
}
}'
Response - Safety Follow-Up Scan
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "safety_integrations_follow_up_scan__v",
"job_id": 307483,
"created_by": "chunter@abcpharma.com",
"created_date": "2023-06-27T20:27:26Z",
"full_scan": true
}
}
See Job Summary / Types (above) for description of this job type.
Endpoint
POST /api/{version}/app/cdm/jobs/start_now
Required Permissions
The following permissions are required to use the Safety Follow-Up Scan Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants the API Access and Manage Jobs permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
Body Parameters
| Name | Description |
|---|---|
study_name |
Name of the Study |
job_type |
Value of safety_integrations_follow_up_scan__v in this case, i.e., for start of this type of job. |
full_scan |
Optional True or false, false is the default if omitted. The parameter dictates whether all safety cases are rescanned ("full_scan": true), or just the safety cases of recently updates Subjects since the last scan. ("full_scan": false) |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
The provided job type: safety_integrations_follow_up_scan__v |
job_id |
Vault Job ID for the job, used to Retrieve Job Status and Retrieve Job Output File |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
full_scan |
Parameter provided, or the default if omitted. |
Cancel Job
Request
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/525010/cancel_now' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "study_data_extract__v",
"job_id": 525010,
"study_name": "ABCP-2022-01_DEV1"
}
}
Notes
- You can cancel a job that is only of In Progress (
in_progress__v) status. - Vault stops the job and moves it into the Canceled (
canceled__v) status. - If you cancel the run of a scheduled job, that job continues to follow the configured schedule, the next round / time period.
- Only Jobs of certain types (see Job Summary / Types) can be canceled.
Endpoint
POST /api/{version}/app/cdm/jobs/{job_id}/cancel_now
Required Permissions
The following permissions are required to use the Cancel Job API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
URI Path Parameters
| Name | Description |
|---|---|
job_id |
The Vault ID of the Job. |
Retrieve Job Status
Request
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/525010' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"job_type": "form_progress_listing__v",
"response": {
"job_id": 525010,
"study_name": "ABCP-2022-01_DEV1",
"status": "in_progress__v",
"created_by": "chunter@abcpharma.com",
"created_date": "2021-10-20T12:14:42Z",
"last_modified_date": "2021-10-20T12:20:42Z"
}
}
Notes
- You can retrieve the current status of a Study Job using its known Vault Job ID.
- The ID is returned in the response to start a Job, or from Retrieve Study Jobs
- Only Jobs of certain types (see Job Summary / Types) can be inspected for current status.
Endpoint
GET /api/{version}/app/cdm/jobs/{job_id}
Required Permissions
The following permissions are required to use the Retrieve Job Status API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
URI Path Parameters
| Name | Description |
|---|---|
job_id |
The Vault ID of the Job. |
Retrieve Job Output File
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/525105/file/content' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
Notes
- You can retrieve the output file of a Study Job (e.g. ZIP CSV, JSON, or other formatted file) using its known Vault Job ID.
- The output file is available for up to thirty (30) after it runs.
- These IDs are part of the response to start a _Job).
- Only jobs of certain types (see Job Summary / Types) allow download of an output file.
Endpoint
GET /api/{version}/app/cdm/jobs/{job_id}/file/content
Required Permissions
The following permissions are required to use the Retrieve Job Output File API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
URI Path Parameters
| Name | Description |
|---|---|
job_id |
The Vault ID of the Job. |
Retrieve Job Log
Request
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/jobs/525105/file/log' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
2023-12-23T00:14:30.655Z Starting execution for job 525105
2023-12-23T00:14:30.723Z Splitting job into chunks for parallel processing.
2023-12-23T00:14:31.438Z 1 chunk(s) created and ready for processing; submitting to queue.
2023-12-23T00:14:32.603Z Processing chunk 4c118fdb-f781-4ada-8f13-68be3779cd2d.
2023-12-23T00:14:33.612Z Successfully processed chunk 4c118fdb-f781-4ada-8f13-68be3779cd2d; marking as completed.
2023-12-23T00:14:34.602Z Received 4c118fdb-f781-4ada-8f13-68be3779cd2d chunk for aggregation; marking as aggregated.
2023-12-23T00:14:34.638Z All chunks for job 525105 processed; beginning aggregation.
2023-12-23T00:14:34.799Z Successfully aggregated completed results for job 525105.
2023-12-23T00:14:34.814Z All chunks and aggregation were completed successfully, marking job as successful.
2023-12-23T00:14:34.944Z
2023-12-23T00:14:34.944Z Job Title: AsyncOperation
2023-12-23T00:14:34.944Z Job Type: ASYNC_OPERATION
2023-12-23T00:14:34.944Z Job Subtype: FORM_PROGRESS_LISTING
2023-12-23T00:14:34.944Z Job Schedule Time: 2023-12-23T00:14:30.000Z
2023-12-23T00:14:34.945Z Job Queue Time: 2023-12-23T00:14:30.000Z
2023-12-23T00:14:34.945Z Job Execution Time: 2023-12-23T00:14:31.000Z
2023-12-23T00:14:34.945Z Job Finish Time: 2023-12-23T00:14:35.000Z
2023-12-23T00:14:34.945Z Job Completion Status: Success (COMPLETED_WITH_SUCCESS)
Notes
- You can retrieve the log file of a Study Job using its known Vault Job ID for up to thirty (30) days after a job is completed.
- The ID is returned in the response to start a Job, or from Retrieve Study Jobs
Endpoint
GET /api/{version}/app/cdm/jobs/{job_id}/file/log
Required Permissions
The following permissions are required to use the Retrieve Job Log API:
- API Access
- Manage Jobs
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json (default) |
URI Path Parameters
| Name | Description |
|---|---|
job_id |
The Vault ID of the Job. |
Users
These endpoints allow for inspection of Study users, and also the update of Study access and/or the overall Vault account of users. Learn more about Study user access in Clinical Data Help.
Retrieve Users
Request
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/users' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 10,
"total": 10
},
"vault_id": 1004329,
"users": [
{
"user_id": "886159",
"user_name": "mary.jones@my-cdms-vaults.com",
"user_email": "mary.jones@mycompany.com",
"user_title": "",
"user_last_name": "Mary",
"user_first_name": "Jones",
"company": "",
"federated_id": "",
"user_language": "en",
"user_locale": "en_US",
"user_timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)",
"security_policy": "Basic",
"security_profile": "cdms_all_access__v",
"activation_date": "2023-09-22",
"created_date": "2023-09-22T14:21:54Z",
"created_by": "Mary Jones",
"last_modified_date": "2023-09-22T15:02:41Z",
"principal_investigator": false,
"active": true,
"vault_owner": false,
"all_studies_access": false,
"last_login": null,
"studies": [
{
"study_environment": "EKE_SFTY_TEST2_DEV1",
"study_role": "CDMS Safety Administrator",
"study_access": "Enabled",
"all_sites_access": true,
"lms_training_status": "Trained",
"ignore_lms_training_status": false
},
{
"study_environment": "EKE_SFTY_TEST1_DEV1",
"study_role": "CDMS Safety Administrator",
"study_access": "Enabled",
"all_sites_access": true,
"lms_training_status": "Trained",
"ignore_lms_training_status": false
}
]
},
{
"user_id": "885131",
"user_name": "tim.young@my-cdms-vaults.com",
"user_email": "tim.young@mycompany.com",
"user_title": "",
"user_last_name": "Tim",
"user_first_name": "Young",
"company": "",
"federated_id": "",
"user_language": "en",
"user_locale": "en_US",
"user_timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)",
"security_policy": "Basic",
"security_profile": "cdms_all_access__v",
"activation_date": "2023-09-22",
"created_date": "2023-09-22T14:21:54Z",
"created_by": "Mary Jones",
"last_modified_date": "2023-09-22T15:02:41Z",
"principal_investigator": false,
"active": true,
"vault_owner": false,
"all_studies_access": false,
"last_login": "2024-01-04T11:12:31Z",
"studies": [
{
"study_environment": "EKE_SFTY_TEST2_DEV1",
"study_role": "CDMS Clinical Research Associate",
"study_access": "Enabled",
"site_access": "101,102,105"
"country_access": "",
"lms_training_status": "Trained",
"ignore_lms_training_status": false
},
{
"study_environment": "EKE_SFTY_TEST1_DEV1",
"study_role": "CDMS Clinical Research Associate",
"study_access": "Enabled",
"site_access": ""
"country_access": "United States,Mexico,Canada",
"lms_training_status": "Not Trained",
"ignore_lms_training_status": true
}
]
},
{
"user_id": "886151",
"user_name": "joe.smith@my-cdms-vaults.com",
"user_email": "joe.smith@mycompany.com",
"user_title": "",
"user_last_name": "Smith",
"user_first_name": "Joe",
"company": "",
"federated_id": "",
"user_language": "en",
"user_locale": "en_US",
"user_timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)",
"security_policy": "Basic",
"security_profile": "cdms_all_access__v",
"activation_date": "2022-06-28",
"created_date": "2022-06-28T19:31:12Z",
"created_by": "Mary Jones",
"last_modified_date": "2022-06-28T19:31:14Z",
"principal_investigator": false,
"active": true,
"vault_owner": false,
"all_studies_access": false,
"last_login": "2024-01-02T13:34:31Z",
"studies": [
{
"access_to_all_environments": true,
"study": "EKE_SFTY_VSCC",
"study_role": "CDMS Lead Data Manager",
"study_access": "Enabled",
"lms_training_status": "",
"ignore_lms_training_status": true
}
]
},
{
"user_id": "883233",
"user_name": "john.johnson@my-cdms-vaults.com",
"user_email": "john.johnson@mycompany.com",
"user_title": "",
"user_last_name": "John",
"user_first_name": "Johnson",
"company": "",
"federated_id": "",
"user_language": "en",
"user_locale": "en_US",
"user_timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)",
"security_policy": "Basic",
"security_profile": "cdms_all_access__v",
"activation_date": "2023-09-22",
"created_date": "2023-09-22T14:20:33Z",
"created_by": "Mary Jones",
"last_modified_date": "2023-09-22T15:02:41Z",
"principal_investigator": false,
"active": true,
"vault_owner": false,
"all_studies_access": true,
"last_login": "2023-12-15T16:05:31Z",
"studies": [
{
"study_role": "CDMS Study Designer",
"study_access": "Enabled",
"lms_training_status": "",
"ignore_lms_training_status": true
}
]
},
{
"user_id": "884213",
"user_name": "jennifer.harrison@my-cdms-vaults.com",
"user_email": "jennifer.harrison@mycompany.com",
"user_title": "",
"user_last_name": "Harrison",
"user_first_name": "Jennifer",
"company": "",
"federated_id": "",
"user_language": "en",
"user_locale": "en_US",
"user_timezone": "(GMT-05:00) Eastern Standard Time (America/New_York)",
"security_policy": "Basic",
"security_profile": "cdms_all_access__v",
"activation_date": "",
"created_date": "2018-07-25T11:23:31Z",
"created_by": "Mary Jones",
"last_modified_date": "2021-03-15T16:05:31Z",
"principal_investigator": false,
"active": true,
"vault_owner": true,
"all_studies_access": false,
"last_login": "2024-01-15T16:05:31Z"
},
:
:
:
]
}
Notes
- Retrieve a list of Users in a single Study, or of many Studies.
- Also retrieve very specific users by username(s).
- Four generic types of users are returned in responses regardless of Study role or Study membership:
- Each has different behavior in their respective entry in the response.
- Review the table below for the different types of users in a EDC Vault, and the specific examples to the right for each
- Each has different behavior in their respective entry in the response.
| Type | Example Users | Description |
|---|---|---|
| Vault Owner | Jennifer Harrison |
Users that have full access to all aspects of the Vault, thus automatically a member of all Studies. Their role in each Study is unlimited. The studies array is omitted from its entry in the response. |
| All Studies User | John Johnson |
Users that have a specific Study role for all Studies in the Vault, now and for any future Study. This is typical in a UAT/Test Vault, where Studies are often deployed, and alleviates the need to re-setup users with every new Study deployment. For the array studies, there is only one entry, and it does not contain study_environment or study property/value since all Studies are granted. These users will always have all site access in their Studies, and as such, all_sites_access, site_access, and country_access are omitted. Only study_role, study_access, lms_training_status, and ignore_lms_training_status are returned, and as a single entry of the array. |
| All Environments User | Joe Smith |
Users that have a specific Study role for all instances of a Study in the Vault, now and for any future instances of that Study. This is also typical in a UAT/Test Vault, where Studies are often deployed, and alleviates the need to re-setup users with every new Study deployment. Example: for a Study in a UAT Vault called ABC-PROT-12345, it will have one or more instances / environments for testing purposes. Suppose they are: ABC-PROT-12345_TST1, ABC-PROT-12345_TST2, and ABC-PROT-12345_TST3. Users of this type will have access into all three with a specific Study role. Any additional deployment for the Study, say ABC-PROT-12345_TST4, will result in automatic access for the user into the newly deployed Study. Similar to the All Studies User, the studies array contains study_role, study_access, lms_training_status, and ignore_lms_training_status. Additionally included is "access_to_all_environments": true and a study value to indicate the Study master name that houses all the instances of the Study. These users will always have all site access in their Studies, and as such all_sites_access, site_access, and country_access are omitted. |
| Study by Study User | Mary Jones and Tim Young |
Users that are the most typical users for EDC, most notably in production Vaults. These users are granted access to one or more Studies each with their own Study role, plus specific Site or Study Country access. |
Endpoint
GET /api/{version}/app/cdm/users
Required Permissions
The following permissions are required to use the Retrieve Users API:
- API Access
- View Users
The CDMS API Read Only and CDMS API Read Write roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Query String Parameters
| Name | Description |
|---|---|
study_name |
Optional Name of the Study |
user_names |
Optional Include a list of usernames (user__v.user_name__v) to retrieve information about specific users. If omitted, Vault returns all Users for the specified Study, or across Studies (if study_name omitted). |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer or zero). If omitted, the default is 0. For example, if the limit is set to 100, and there are 150 possible records, an offset of 0 returns records 0 through 99 (zero based). Then, offset of 100 would return records 100 through 149. More information on pagination is in the section Pagination |
Response Details
On SUCCESS, Vault returns a list of users with properties on their Vault level account, plus any Study access:
| Array | Name | Description |
|---|---|---|
vault_id |
The unique Vault ID, i.e., one currently connected with. | |
users |
user_id |
Value is across all Vaults / domains |
users |
user_name |
E.g., joe.smith@eke.com |
users |
user_email |
|
users |
user_title |
|
users |
user_last_name |
|
users |
user_first_name |
|
users |
users/company |
|
users |
federated_id |
This value is the username in a system the user is using SSO for to login to the Vault. The security policy must be set appropriately for this value to line up. (multiple SSO policies can reside in one Vault) |
users |
user_language |
E.g., en, fr, de, etc. |
users |
user_locale |
E.g., en_US, en_AU, etc. |
users |
user_timezone |
Full label of the user’s time zone |
users |
security_policy |
The security policy name value, e.g. Basic or Customer Network SSO, etc. |
users |
security_profile |
The overall Vault security profile for the user. In EDC, this is most often a very generice profile, with Study level role dictating the actual permissions of a user in the Study. |
users |
activation_date |
If there is an activation date, shown as yyyy-MM-dd format. Otherwise the line is omitted |
users |
created_date |
Format yyyy-MM-ddThh:ss:mmZ, UTC date time |
users |
created_by |
The user that created this account (Vault level account) |
users |
last_modified_date |
Format yyyy-MM-ddThh:ss:mmZ, UTC date time |
users |
principal_investigator |
true/false When choosing 'Add as Principal Investigator' from the UI, a user can also be a PI for tagging to a specific site. If the “Enable Person and Just in Time VeevaID User Creation” feature flag is on, a user is recognized as a Principal Investigator (PI) on a site only if they are referenced in the person__sys field. If the flag is off, they must be referenced in the person__v field instead. |
users |
active |
The status of the overall Vault account in the Vault (no specific Study) |
users |
vault_owner |
If the user is a Vault Owner, they have access to all Studies (need no specific Study access). These users returned will not have the studies array in their response entry. |
users |
all_studies_access |
When a user is set to have access to all Studies, now and in the future. (e.g. a Sandbox or Test Vault). Users with true will always have all sites access in the Studies. See also the All Studies User described above. |
users |
last_login |
The last login to the Vault for this user (no specific Study). Format yyyy-MM-ddThh:ss:mmZ, UTC date time |
users/studies |
access_to_all_environments |
When true, the user has access to all Study instances, and the Study master name is noted in the study line noted below, and will not yield a study_environment line. This user will also always have all sites access in the Study instances. When the user does not have all environments access, the line is omitted. See also the All Environments User described above. |
users/studies |
study_environment |
The Study instance label, e.g. ABC-2022-01_DEV1. Only the Study by Study User described above will contain this property / value. |
users/studies |
study |
The Study master name, e.g. ABC-2022-01, where ABC-2022-01_DEV1 is the development Study, ABC-2022-01_TST1, ABC-2022-01_TST2, .. are the test Vault Studies. Only those with "access_to_all_environments": true will have this property / value. See also the All Environments User described above. |
users/studies |
study_role |
The Study role name in the Study (Study by Study User), all instances of Study (All Environments User), or all Studies (All Studies User) |
users/studies |
study_access |
Values of Enabled or Disabled, the status of the entry, it for a single Study (Study by Study User), multiple instances of a Study (All Environments User), or all Studies (All Studies User) |
users/studies |
all_sites_access |
When the value is true, the user has access to all sites in the Study. If false, the line is omitted from the return, and instead the Site level access is shown in site_access or country_access |
users/studies |
site_access |
Comma list of Sites the user has access to, usually a single entry when used. Data entry users (CRC / PI) are the common roles for this access line. The line is omitted when "all_sites_access": true, or when the user is of type All Studies User or All Environments User. |
users/studies |
country_access |
Comma list of Study Countries the user has access to. That is, the Sites of those listed countries. The line is omitted when "all_sites_access": true, or when the user is of type All Studies User or All Environments User. |
users/studies |
lms_training_status |
Values are empty, Trained or Not Trained. This appears for Study by Study User, All Studies User, or All Environments User. |
users/studies |
ignore_lms_training_status |
When this value is true, the LMS training status on that Study is ignored (e.g. Not Trained). That is, the user is allowed into the Study per this override - common to development / test Study users. This appears for Study by Study User, All Studies User, or All Environments User. |
Upload Users (CSV)
Request
curl -L -X PUT 'https://my-vault.veevavault.com/api/v24.1/app/cdm/users' \
-H 'Authorization: {SESSION_ID}' \
-F 'append_site_country_access="false"' \
-F 'import_file=@"/Users/joe_smith/user-import-test.csv"'
Response
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "EDC Import",
"job_id": 581803,
"status": "Queued",
"created_by": "chunter@abcpharma.com",
"created_date": "2022-02-14T23:37:17Z"
}
}
Notes
- This endpoint is used to create or update users in a Study or Studies.
- Each row of the uploaded CSV is for a user to create or make updates to. This can be the creation of both the Vault account and access into a specific Study or Studies, or just the creation or update of Study specific access for an existing User.
- For deactivation at a Study level, this endpoint is also used. Specifically, a value of Disabled in the Study Access column for the specific User to deactivate. A value of Enabled can be used to re-activate or initially activate an existing Study user that is currently inactive.
- Vault or domain activation or deactivation - see the next few sections
- If ANY of the rows fail, the return response will enumerate those rows with issues. A job does NOT start in such cases. A list of possible errors and warnings for user import is available on Clinical Data Help
- Once a job successfully starts, the job ID is returned. Check the job status using:
<your Vault URL>/api/v24.1/services/jobs/{job_id}
- Once the job is complete, consider using Retrieve Users to verify the state of each of the users created and/or updated.
- Information about the upload of Users via CSV via the UI are also available here: Importing Users
Preparing the Import File
Use this template file to create your CSV. Detailed explanations for the required columns are available on Clinical Data Help.
The CSV file must meet the following general requirements:
- The CSV file must be smaller than 20 mb.
- Use CSV UTF-8 encoding, or don't use any special characters.
- The CSV file must follow standard CSV format.
- The matching of values in the import file to those values in EDC is case-sensitive.
- The CSV attempted is checked for correct column header names first, then cell by cell validation of values.
- CSV Column names are case sensitive, but can be in any order
Endpoint
PUT /api/{version}/app/cdm/users
Required Permissions
The following permissions are required to use the Upload Users (CSV) API:
- API Access
- View Users
- Edit Users
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
multipart/form-data |
Body Parameters
| Name | Description |
|---|---|
import_file |
Provide the path to the import CSV file. |
append_site_country_access |
Optional When omitted, false is assumed. Use true to append Site and Country access ONLY for existing users. Example - a user has existing Site access of 101,102. If a CSV is uploaded with line corresponding to the user and only 103 is in the Site Access column, the result is the user having overall Site access of 101,102,103. With this parameter false in the example, the user would be updated to just Site 103. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
Value is always EDC Import, the job label for the User import |
job_id |
Vault Job ID for the job that will start |
status |
Value is always Queued, i.e., about to start |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
Upload Users (JSON)
Request - Two users
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/users_json' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"append_site_country_access": false,
"users": [
{
"User Name": "joe.smith@my-cdms-vaults.com",
"Email": "joe.smith@veeva.com",
"Title": "Mr.",
"First Name": "Joe",
"Last Name": "Smith",
"Company": "Sponsor ABCXYZ",
"Language": "en",
"Locale": "en_US",
"Timezone": "(GMT-08:00) Pacific Standard Time (America/Los_Angeles)",
"Security Policy": "Basic",
"Federated ID": "",
"Activation Date": "10/15/2023",
"Send Welcome Email": "Yes",
"Add as Principal Investigator": "No",
"Study": "ABCP-2023-001",
"Study Environment": "ABCP-2023-001_TST5",
"Access to All Environments": "No",
"Cross Study Role": "",
"Study Access": "Enabled",
"Study Role": "CDMS Lead Data Manager",
"Access to All Sites": "Yes",
"Country Access": "",
"Site Access": "",
"Ignore LMS Status": "No",
"Domain Administrator": "No",
"Service Availability Notifications": "No",
"Product Announcement Emails": "No",
"Status": "Active",
"Bypass Veeva ID Check": "No"
},
{
"User Name": "mary.jones@my-cdms-vaults.com",
"Email": "mary.jones@veeva.com",
"Title": "",
"First Name": "Mary",
"Last Name": "Jones",
"Company": "Cary General Hospital",
"Language": "en",
"Locale": "en_US",
"Timezone": "(GMT-08:00) Pacific Standard Time (America/Los_Angeles)",
"Security Policy": "Basic",
"Federated ID": "",
"Activation Date": "10/15/2023",
"Send Welcome Email": "Yes",
"Add as Principal Investigator": "No",
"Study": "ABCP-2023-001",
"Study Environment": "ABCP-2023-001_TST5",
"Access to All Environments": "No",
"Cross Study Role": "",
"Study Access": "Enabled",
"Study Role": "CDMS Clinical Research Coordinator",
"Access to All Sites": "No",
"Country Access": "",
"Site Access": "101",
"Ignore LMS Status": "No",
"Domain Administrator": "No",
"Service Availability Notifications": "No",
"Product Announcement Emails": "No",
"Status": "Active",
"Bypass Veeva ID Check": "No"
}
]
}
'
Response - Success - job started to process users
{
"responseStatus": "SUCCESS",
"response": {
"job_type": "EDC Import",
"job_id": 581803,
"status": "Queued",
"created_by": "chunter@abcpharma.com",
"created_date": "2023-06-14T23:37:17Z"
}
}
Notes
- This endpoint is used to create or update users in a Study or Studies.
- Instead of a CSV file as the body of the request, JSON formatted body for the request.
- Use cases include the creation of both the Vault account and access into a specific Study or Studies, or just the creation or update of Study specific access for an existing User.
- For deactivation at a Study level, the endpoint is also used. Specifically, a value of Disabled in the Study Access JSON node for the specific User to deactivate. A value of Enabled can be used to re-activate or initially activate an existing Study user that is currently inactive.
- Vault or domain activation or deactivation - see the next few sections
- If ANY of the entries in the request fail, the return response will enumerate those entries with issues. A job does NOT start in such cases. A list of possible errors and warnings for user import is available on Clinical Data Help
- Once a job successfully starts, the job ID is returned. Check the job status using:
<your Vault URL>/api/v24.1/services/jobs/{job_id}
- This is a general check on any Vault Job, by its ID. Once the job is complete, consider using Retrieve Users to verify the state of each of the users created and/or updated.
- Information about the upload of Users via CSV via the UI is also available here: Importing Users. The API attempt through CSV or JSON goes through all of the same validation checks.
Endpoint
POST /api/{version}/app/cdm/users_json
Required Permissions
The following permissions are required to use the Upload Users (JSON) API:
- API Access
- View Users
- Edit Users
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
multipart/form-data |
Body Parameters
| Name | Description |
|---|---|
import_file |
Provide the path to the import CSV file. |
append_site_country_access |
Optional When omitted, false is assumed. Use true to append Site and Country access ONLY for existing users. Example - a user has existing Site access of 101,102. If a CSV is uploaded with line corresponding to the user and only 103 is in the Site Access column, the result is the user having overall Site access of 101,102,103. With this parameter false in the example, the user would be updated to just Site 103. |
Response Details
On SUCCESS, Vault returns the following information on successful start of the job:
| Name | Description |
|---|---|
job_type |
Value is always EDC Import, the job label for the User import |
job_id |
Vault Job ID for the job that will start |
status |
Value is always Queued, i.e., about to start |
created_by |
User Name of the user who started the job. |
created_date |
Datetime the job was started. (UTC) |
Inactivate User
Request - Inactivate User in a Vault
$ curl -X DELETE -H "Authorization: {SESSION_ID}" \
https://my-vault.veevavault.com/api/v24.1/objects/users/25001
Request - Inactivate User in All Vaults of the Domain
$ curl -X DELETE -H "Authorization: {SESSION_ID}" \
https://my-vault.veevavault.com/api/v24.1/objects/users/25001?domain=true
Response - (either)
{
"responseStatus": "SUCCESS",
"id": 25001
}
Notes
- Use this endpoint ONLY to inactivate a user account, Vault or domain wise.
- For the deactivation of just a specific Study, use the Upload Users endpoint, but with a line for the user indicating Disabled Study status.
- WARNING: This endpoint with domain = true will inactivate the user across ALL Vaults they otherwise have access into. The use of domain = false (or omitting the domain parameter) will inactivate the user in just the Vault targeted. This will of course deactivate the user in all Studies in that Vault they otherwise had access to.
- This endpoint is a mirror of the main Vault endpoint for inactivating a user : Vault Help
- To inactivate a user across a domain, your account must be a Domain Admin
Endpoint
DELETE /api/{version}/objects/users/{user_id}
Required Permissions
The following permissions are required to use the Inactivate Users API:
- API Access
- View Users
- Edit Users
The CDMS API Read Write role grants these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) or application/xml |
URI Path Parameters
| Name | Description |
|---|---|
user_id |
The Vault unique ID for the user. See Retrieve Users above to retrieve those ID(s). |
Query String Parameters
| Name | Description |
|---|---|
domain |
Optional When true, this disables the user account in all Vaults in the domain. When omitted, a value of false is assumed, i.e., inactivate the user just in the one Vault. |
Activate User - Domain Level
Request - Activate User at Domain-Level
$ curl -X PUT -H "Authorization: {SESSION_ID}" \
-H "Content-Type: application/x-www-form-urlencoded" \
-d "domain_active__v=true" \
https://my-vault.veevavault.com/api/v24.1/objects/users/25001
Response - Activate User at Domain-Level
{
"responseStatus": "SUCCESS",
"id": 25001
}
Notes
- Use this endpoint ONLY to activate a user account that is currently inactive on the domain (all Vaults in the domain)
- This action must be performed first, prior to more actions to activate in specific Vaults, then more to activate specific Studies (where necessary)
- This endpoint is a mirror of the main Vault endpoint for updating a user : Vault Help
- To activate a user across a domain, your account must be a Domain Admin
Required Permissions
The following permissions are required to use the Activate Users - Domain Level API:
- API Access
- View Users
- Edit Users
The CDMS API Read Write role grants these permissions.
Your user account must also be a Domain Administrator to make changes at the domain level.
Endpoint
PUT /api/{version}/objects/users/{user_id}
Headers
| Name | Description |
|---|---|
Content-Type |
application/x-www-form-urlencoded |
Accept |
application/json (default) or application/xml |
URI Path Parameters
| Name | Description |
|---|---|
user_id |
The Vault unique ID for the user. See Retrieve Users above to retrieve those ID(s). |
Body Parameters
| Name | Description |
|---|---|
domain_active__v |
Encoded parameter, use true. This is the field name on the user object in Vault. The endpoint is a generic endpoint to update various fields of the user (at Vault level). In this case/use, one just uses just the domain active, i.e., setting to true for re-activation of a user. |
Activate User - Vault(s)
Request - Activate User at Vault Level
$ curl -X POST 'https://my-vault.veevavault.com/api/v24.1/vobjects/user__sys/25001/actions/Objectlifecyclestateuseraction.user__sys.inactive_state__sys.change_state_to_active_useraction__sys' \
-H "Authorization: {SESSION_ID}"
Response - Activate User at Vault Level
{
"responseStatus": "SUCCESS"
}
This endpoint is the recommended method for activating inactive site and sponsor user accounts, including those for non-VeevaIDs users. It is only to be used to activate a user account that is currently inactive on a specific Vault. This action must be performed first, prior to specific Study activations (where necessary).
Required Permissions
The following permissions are required to use the Activate Users - Vault Level API:
- API Access
- View Users
- Edit Users
The CDMS API Read Write role grants these permissions.
Endpoint
POST /api/{version}/vobjects/user__sys/{user_id}/actions/Objectlifecyclestateuseraction.user__sys.inactive_state__sys.change_state_to_active_useraction__sys
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) or application/xml |
URI Path Parameters
| Name | Description |
|---|---|
user_id |
The Vault unique ID for the user. See Retrieve Users above to retrieve those ID(s). |
Retrieve Study Roles
Request - Retrieve study roles
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/studyroles' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - Standard and configured roles in Vault
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 47,
"total": 47
},
"studyroles": [
{
"id": "0AR00000000I001",
"role_api_name": "cdms_api_read_only__v",
"role_name": "CDMS API Read Only",
"role_team": "other__v",
"accept_closeout_pdf": false,
"add_casebook": false,
"answer_query": false,
"answer_third_party_queries": false,
"api_access": true,
"approve_code": false,
"approve_import": false,
"approve_lab_normals": false,
"assessments_tab": false,
"assign_code": false,
"browse_view": false,
"cdb_tools": false,
"close_all_queries": false,
"close_query": false,
"coder_tab": false,
"coder_tools_tab": false,
"configure_cdb": false,
"configure_randomization": false,
"copy_study_data_to_ppt": false,
"create_export_definition": false,
"create_listing": false,
"create_protocol_deviations": false,
"create_view": false,
"data_entry": false,
"data_entry_tab": false,
"delete_casebook": false,
"delete_export_definition": false,
"delete_listing": false,
"delete_view": false,
"design_library": false,
"design_study": false,
"download_import_package": false,
"edc_tools_tab": false,
"edit_classification": false,
"edit_clinical_assessments": false,
"edit_cql": false,
"edit_dmr": false,
"edit_form_linking": false,
"edit_integration_mappings": false,
"edit_lab_analyte_library": false,
"edit_lab_locations_and_normals": false,
"edit_protocol_deviations": false,
"edit_sdv": false,
"edit_study_settings": false,
"execute_query_rules": false,
"freeze_data": false,
"generate_blank_pdf": false,
"generate_closeout_pdf": false,
"generate_csv": false,
"generate_detail_pdf": false,
"generate_export_package": false,
"invalidate_randomization": false,
"lab_mass_update": false,
"labs_tab": false,
"library_tab": false,
"lock_data": false,
"manage_amendments": false,
"manage_assessments": false,
"manage_bulk_lock_and_freeze": false,
"manage_coder_study_settings": false,
"manage_coding_lists": false,
"manage_data_and_definition_export": false,
"manage_email_group_assignment": false,
"manage_ftp": false,
"manage_jobs": false,
"manage_key_mappings": false,
"manage_lab_study_settings": false,
"manage_lab_units_and_codelists": false,
"manage_learning": false,
"manage_randomization_list": false,
"manage_review_plan_assignment_criteria": false,
"manage_review_plan_manual_assignment": false,
"manage_review_plans_assignment": false,
"manage_safety_configuration": false,
"manage_safety_integrations": false,
"manage_site_lab_assignment": false,
"manage_site_vault_settings": false,
"manage_sources": false,
"manage_study_countries": false,
"manage_study_deployments": false,
"manage_study_lock": false,
"manage_study_priority": false,
"manage_study_roles": false,
"manage_study_sites": false,
"manage_unblinding_rules": false,
"manage_users": false,
"modify_listing": false,
"modify_view": false,
"notify_sites_of_closeout": false,
"open_query": false,
"protocol_deviations_tab": false,
"public_access": false,
"randomization_list_actions": false,
"randomization_tab": false,
"randomize_subject": false,
"reports_dashboards_tab": false,
"restricted_data_access": false,
"reveal_treatment": false,
"review_closeout_pdf": false,
"review_tab": false,
"safety_integrations_tab": false,
"schedule_reports": false,
"sign": false,
"studio_tab": false,
"system_tools_tab": false,
"unrestrict_randomization_data": false,
"vault_configuration_report": false,
"view_admin": false,
"view_all_lab_settings": false,
"view_bulk_lock_and_freeze": false,
"view_casebook": true,
"view_classification": true,
"view_clinical_assessments": false,
"view_code": true,
"view_dmr": false,
"view_export": false,
"view_export_packages": false,
"view_form_linking": false,
"view_import": false,
"view_integration_mappings": false,
"view_lab_analyte_library": false,
"view_lab_locations_and_normals": false,
"view_library": true,
"view_listings": false,
"view_protocol_deviations": false,
"view_queries": false,
"view_query": true,
"view_randomization_enrollment": false,
"view_randomization_kit_device": false,
"view_restricted_randomization_enrollment": false,
"view_safety_integrations": false,
"view_sdv": false,
"view_selected_cdb_query_listings": false,
"view_selected_listings": false,
"view_study_design": true,
"view_study_sites": true,
"view_users": true,
"workbench_tab": false
},
{
"id": "0AR00000000I002",
"role_api_name": "cdms_api_read_write__v",
"role_name": "CDMS API Read Write",
"role_team": "other__v",
"accept_closeout_pdf": false,
"add_casebook": true,
"answer_query": true,
"answer_third_party_queries": false,
"api_access": true,
"approve_code": false,
"approve_import": false,
"approve_lab_normals": false,
"assessments_tab": true,
"assign_code": true,
"browse_view": false,
"cdb_tools": false,
"close_all_queries": false,
"close_query": true,
"coder_tab": true,
"coder_tools_tab": true,
"configure_cdb": false,
"configure_randomization": false,
"copy_study_data_to_ppt": false,
"create_export_definition": false,
"create_listing": false,
"create_protocol_deviations": false,
"create_view": false,
"data_entry": true,
"data_entry_tab": true,
"delete_casebook": false,
"delete_export_definition": false,
"delete_listing": false,
"delete_view": false,
"design_library": true,
"design_study": true,
"download_import_package": false,
"edc_tools_tab": true,
"edit_classification": true,
"edit_clinical_assessments": false,
"edit_cql": false,
"edit_dmr": false,
"edit_form_linking": false,
"edit_integration_mappings": false,
"edit_lab_analyte_library": false,
"edit_lab_locations_and_normals": false,
"edit_protocol_deviations": false,
"edit_sdv": false,
"edit_study_settings": false,
"execute_query_rules": false,
"freeze_data": false,
"generate_blank_pdf": false,
"generate_closeout_pdf": false,
"generate_csv": false,
"generate_detail_pdf": false,
"generate_export_package": false,
"invalidate_randomization": false,
"lab_mass_update": false,
"labs_tab": true,
"library_tab": true,
"lock_data": false,
"manage_amendments": false,
"manage_assessments": false,
"manage_bulk_lock_and_freeze": false,
"manage_coder_study_settings": false,
"manage_coding_lists": false,
"manage_data_and_definition_export": false,
"manage_email_group_assignment": true,
"manage_ftp": false,
"manage_jobs": true,
"manage_key_mappings": false,
"manage_lab_study_settings": false,
"manage_lab_units_and_codelists": false,
"manage_learning": false,
"manage_randomization_list": false,
"manage_review_plan_assignment_criteria": false,
"manage_review_plan_manual_assignment": false,
"manage_review_plans_assignment": false,
"manage_safety_configuration": false,
"manage_safety_integrations": false,
"manage_site_lab_assignment": false,
"manage_site_vault_settings": false,
"manage_sources": false,
"manage_study_countries": false,
"manage_study_deployments": false,
"manage_study_lock": false,
"manage_study_priority": false,
"manage_study_roles": false,
"manage_study_sites": true,
"manage_unblinding_rules": false,
"manage_users": true,
"modify_listing": false,
"modify_view": false,
"notify_sites_of_closeout": false,
"open_query": true,
"protocol_deviations_tab": true,
"public_access": false,
"randomization_list_actions": false,
"randomization_tab": true,
"randomize_subject": false,
"reports_dashboards_tab": true,
"restricted_data_access": false,
"reveal_treatment": false,
"review_closeout_pdf": false,
"review_tab": true,
"safety_integrations_tab": true,
"schedule_reports": false,
"sign": false,
"studio_tab": true,
"system_tools_tab": true,
"unrestrict_randomization_data": false,
"vault_configuration_report": false,
"view_admin": false,
"view_all_lab_settings": false,
"view_bulk_lock_and_freeze": false,
"view_casebook": true,
"view_classification": true,
"view_clinical_assessments": false,
"view_code": true,
"view_dmr": false,
"view_export": false,
"view_export_packages": false,
"view_form_linking": false,
"view_import": false,
"view_integration_mappings": false,
"view_lab_analyte_library": false,
"view_lab_locations_and_normals": false,
"view_library": true,
"view_listings": false,
"view_protocol_deviations": false,
"view_queries": false,
"view_query": true,
"view_randomization_enrollment": false,
"view_randomization_kit_device": false,
"view_restricted_randomization_enrollment": false,
"view_safety_integrations": false,
"view_sdv": false,
"view_selected_cdb_query_listings": false,
"view_selected_listings": false,
"view_study_design": true,
"view_study_sites": true,
"view_users": true,
"workbench_tab": true
},
{
"id": "0AR000000000706",
"role_api_name": "cdms_lead_data_manager__v",
"role_name": "CDMS Lead Data Manager",
"role_team": "data_management__v",
"accept_closeout_pdf": false,
"add_casebook": false,
"answer_query": false,
"answer_third_party_queries": false,
"api_access": true,
"approve_code": false,
"approve_import": true,
"approve_lab_normals": false,
"assessments_tab": false,
"assign_code": false,
"browse_view": true,
"cdb_tools": true,
"close_all_queries": true,
"close_query": true,
"coder_tab": false,
"coder_tools_tab": false,
"configure_cdb": false,
"configure_randomization": false,
"copy_study_data_to_ppt": false,
"create_export_definition": true,
"create_listing": true,
"create_protocol_deviations": true,
"create_view": false,
"data_entry": false,
"data_entry_tab": false,
"delete_casebook": false,
"delete_export_definition": false,
"delete_listing": false,
"delete_view": false,
"design_library": false,
"design_study": false,
"download_import_package": true,
"edc_tools_tab": true,
"edit_classification": false,
"edit_clinical_assessments": false,
"edit_cql": true,
"edit_dmr": true,
"edit_form_linking": false,
"edit_integration_mappings": true,
"edit_lab_analyte_library": false,
"edit_lab_locations_and_normals": false,
"edit_protocol_deviations": true,
"edit_sdv": false,
"edit_study_settings": true,
"execute_query_rules": true,
"freeze_data": true,
"generate_blank_pdf": true,
"generate_closeout_pdf": true,
"generate_csv": true,
"generate_detail_pdf": true,
"generate_export_package": true,
"invalidate_randomization": false,
"lab_mass_update": true,
"labs_tab": true,
"library_tab": false,
"lock_data": true,
"manage_amendments": true,
"manage_assessments": true,
"manage_bulk_lock_and_freeze": true,
"manage_coder_study_settings": false,
"manage_coding_lists": false,
"manage_data_and_definition_export": false,
"manage_email_group_assignment": true,
"manage_ftp": true,
"manage_jobs": true,
"manage_key_mappings": false,
"manage_lab_study_settings": true,
"manage_lab_units_and_codelists": true,
"manage_learning": true,
"manage_randomization_list": false,
"manage_review_plan_assignment_criteria": false,
"manage_review_plan_manual_assignment": false,
"manage_review_plans_assignment": true,
"manage_safety_configuration": false,
"manage_safety_integrations": true,
"manage_site_lab_assignment": true,
"manage_site_vault_settings": true,
"manage_sources": true,
"manage_study_countries": true,
"manage_study_deployments": false,
"manage_study_lock": true,
"manage_study_priority": true,
"manage_study_roles": true,
"manage_study_sites": true,
"manage_unblinding_rules": true,
"manage_users": false,
"modify_listing": true,
"modify_view": false,
"notify_sites_of_closeout": true,
"open_query": true,
"protocol_deviations_tab": true,
"public_access": true,
"randomization_list_actions": false,
"randomization_tab": true,
"randomize_subject": false,
"reports_dashboards_tab": true,
"restricted_data_access": true,
"reveal_treatment": false,
"review_closeout_pdf": false,
"review_tab": true,
"safety_integrations_tab": true,
"schedule_reports": true,
"sign": false,
"studio_tab": false,
"system_tools_tab": true,
"unrestrict_randomization_data": false,
"vault_configuration_report": false,
"view_admin": false,
"view_all_lab_settings": true,
"view_bulk_lock_and_freeze": true,
"view_casebook": true,
"view_classification": false,
"view_clinical_assessments": false,
"view_code": true,
"view_dmr": true,
"view_export": true,
"view_export_packages": true,
"view_form_linking": true,
"view_import": true,
"view_integration_mappings": true,
"view_lab_analyte_library": true,
"view_lab_locations_and_normals": true,
"view_library": false,
"view_listings": true,
"view_protocol_deviations": true,
"view_queries": true,
"view_query": true,
"view_randomization_enrollment": true,
"view_randomization_kit_device": false,
"view_restricted_randomization_enrollment": false,
"view_safety_integrations": true,
"view_sdv": true,
"view_selected_cdb_query_listings": false,
"view_selected_listings": false,
"view_study_design": false,
"view_study_sites": true,
"view_users": false,
"workbench_tab": true
},
:
:
:
(etc. - all other roles...)
]
}
Notes
- Retrieve Study roles in the Vault.
- The roles returned include the standard/template and configured/custom roles
- The response details - role name - is used in Upload Users (CSV) and Upload Users (JSON).
- As new releases come out, the list of permissions in the system will grow (e.g.
add_casebook__v,view_sdv__v, etc). The response is not API version specific.
Endpoint
GET /api/{version}/app/cdm/studyroles
Required Permissions
The following permission is required to use the Retrieve Study Roles API:
API Access
The CDMS API Read Write and CDMS API Read Only roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Type | Required | Description |
|---|---|---|---|
limit |
URL parameter | No | Standard Vault behavior for limit - to limit the return body. Depending on offset used, one receives the ones starting at that point. The maximum is 1000. If the API user passed a negative integer or zero, it is converted to default (1000). |
offset |
URL parameter | No | Standard Vault behavior for offset - to limit the return body. Depending on offset used, one receives the ones starting at that point. The maximum is 2147483647 (large integer). If the API user passed a negative integer, it is converted to default (0). |
Response Details
| Array | Name | Description |
|---|---|---|
studyroles |
id |
Vault internal ID for the Study role. |
studyroles |
role_api_name |
Study role API name. |
studyroles |
accept_closeout_pdf |
Study role acceptance closeout PDF. |
Retrieve Email Groups
Request - Two email groups
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_groups?study=ABCP-2023-001_TST5' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - Two email groups
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"email_groups": [
{
"id": "XYZ0000000123",
"name": "Main Study Alerts",
"members": 2
},
{
"id": "XYZ0000000125",
"name": "Safety Alerts",
"members": 0
}
]
}
Notes
- Retrieve information about email groups.
- This API tells you if a group exists, including groups without members.
- It does not provide member details. (see Retrieve Email Group Members)
Endpoint
GET /api/{version}/app/cdm/email_groups
Required Permissions
The following permission is required to use the Retrieve Email Groups API:
API Access
The CDMS API Read Write and CDMS API Read Only roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Type | Required | Description |
|---|---|---|---|
study |
URL parameter | Yes | Match of study__v.label__v |
limit |
URL parameter | No | Standard Vault behavior for limit - to limit the return body. Depending on offset used, one receives the ones starting at that point. The maximum is 1000. If the API user passed a negative integer or zero, it is converted to default (1000). |
offset |
URL parameter | No | Standard Vault behavior for offset - to limit the return body. Depending on offset used, one receives the ones starting at that point. The maximum is 2147483647 (large integer). If the API user passed a negative integer, it is converted to default (0). |
Response Details
On SUCCESS, Vault returns information about email groups.
| Array | Name | Description |
|---|---|---|
email_groups |
id |
Vault internal ID for the email group. |
email_groups |
name |
Name of the email group. |
email_groups |
members |
Count of existing members in the email group. |
Retrieve Email Group Members
Request - Email group with members (by ID)
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_groups?study=ABCP-2023-001_TST5&email_group_id=XYZ0000000123' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - Email group with members (by ID)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"users": [
{
"user_id": 212333,
"user_name": "joe.smith@sb-abcpharma.com",
"user_email": "joe.smith@veeva.com",
"user_last_name": "Smith",
"user_first_name": "Joe"
},
{
"user_id": 212345,
"user_name": "mary.jones@sb-abcpharma.com",
"user_email": "mary.jones@veeva.com",
"user_last_name": "Jones",
"user_first_name": "Mary"
}
]
}
Request - Group with no members (by name)
curl -L -X GET 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_groups?study=ABCP-2023-001_TST5&email_group_name=Safety_Alerts' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - Group with no members (by name)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 0,
"total": 0
},
"users": []
}
Notes
- Get users who are members of a single email group.
- For existing email groups in the Study, see Retrieve Email Groups
Endpoint
GET /api/{version}/app/cdm/email_group_members
Required Permissions
The following permission is required to use the Retrieve Email Group Members API:
API Access
The CDMS API Read Write and CDMS API Read Only roles grant this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Type | Required | Description |
|---|---|---|---|
study |
URL parameter | Yes | Match of study__v.label__v |
email_group_name |
URL parameter | Yes | Match to email_group_def__v.name__v. Name or ID is required. |
email_group_id |
URL parameter | Yes | Match to email_group_def__v.id. Name or ID is required. If both are provided, the ID is used for lookup, as if the name was not passed. |
limit |
URL parameter | No | Standard Vault behavior for limit to limit within the return body. Depending on the offset used, you receive the ones starting at that point. The maximum is 1000. If the API user passes a negative integer or zero, it is converted to the default (1000). |
offset |
URL parameter | No | Standard Vault behavior for offset to limit within the return body. Depending on the offset used, you receive the ones starting at that point. The maximum is 2147483647 (large integer). If the API user passes a negative integer, it is converted to the default (0). |
Response Details
On SUCCESS, Vault returns members of a single email group.
| Array | Name | Description |
|---|---|---|
users |
user_id |
Vault internal ID of the user. |
users |
user_name |
User name. |
users |
user_email |
User's email address. |
users |
user_last_name |
User's last name. |
users |
user_first_name |
User's first name. |
Update Email Group Members
Request - Remove existing members and set specified users
curl -L -X PUT 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_group_members' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
-d '{
"study": "ABCP-2022-01_DEV1",
"email_group_name": "Main Study Alerts",
"users": ["212333", "212345"]
}'
Response - Remove existing members and set specified users
{
"responseStatus": "SUCCESS",
"study": "ABCP-2022-01_DEV1",
"email_group_id": "XYZ0000000123",
"email_group_name": "Main Study Alerts",
"members": 2
}
Request - Add one user to an existing group
curl -L -X POST 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_group_members' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
-d '{
"study": "ABCP-2022-01_DEV1",
"email_group_name": "Main Study Alerts",
"users": ["212336"]
}'
Response - Add one user to an existing group
{
"responseStatus": "SUCCESS",
"study": "ABCP-2022-01_DEV1",
"email_group_id": "XYZ0000000123",
"email_group_name": "Main Study Alerts",
"members": 12,
"users_added": 1
}
Request - Remove a specific user from a group
curl -L -X DELETE 'https://my-vault.veevavault.com/api/v24.1/app/cdm/email_group_members' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
-d '{
"study": "ABCP-2022-01_DEV1",
"email_group_name": "Main Study Alerts",
"users": ["212347"]
}'
Response - Remove a specific user from a group
{
"responseStatus": "SUCCESS",
"study": "ABCP-2022-01_DEV1",
"email_group_id": "XYZ0000000123",
"email_group_name": "Main Study Alerts",
"members": 11,
"users_removed": 1
}
Notes
- Update the users of an email group.
- Actions possible - remove and replace users, delete all users, and add specific users.
- For the different actions:
- PUT: Deletes all members and adds specified users as members (i.e., a complete replacement of the members with the supplied array of users).
- POST: Adds specified users to an email group as members. If the users are already members of the group, no change occurs.
- DELETE: Delete specified group members. No action occurs for non-members.
- Learn more about Email Groups inClinical Data Help.
Endpoints
PUT /api/{version}/app/cdm/email_group_members
POST /api/{version}/app/cdm/email_group_members
DELETE /api/{version}/app/cdm/email_group_members
Required Permissions
The following permission is required to use the Update Email Group Members API:
API Access
The CDMS API Read Write role grants this permission.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query String Parameters
| Name | Type | Required | Description |
|---|---|---|---|
study |
String | Yes | Match of study__v.label__v. |
email_group_name |
String | No | Match to email_group_def__v.name__v. Name or ID is required. |
email_group_id |
String | No | Match to email_group_def__v.id. Name or ID is required. |
users |
JSON array of string | Yes | The users to act on. Entries are Vault user IDs. |
Response Details
| Name | Description |
|---|---|
email_group_id |
Vault internal ID for the email group. |
email_group_name |
Name of the email group. |
members |
Count of users in the email group, after PUT, POST, or DELETE. |
users_added |
Count of actual users added, during POST only. The value considers users who were already present and those included in the request. |
users_removed |
Count of actual users removed, during DELETE only. The value considers users who were previously absent from the group as well as those included in the request. |
Study Design
Studies are designed using design level components that mirror the data levels:

- Specifically, a Casebook Version is comprised of:
- Event Group Definition (top level)
- .. then.. -> Event Definition
- .. then.. -> Form Definition
- .. then.. -> Item Group Definition
- .. then.. -> Item Definition
- .. finally, with Codelist / Unit Codelist Definitions (used by Item Definitions)
- Each casebook version yields an ordered structure of the various levels, i.e. the intended schedule of a Subject in the Study.
- Once a Study is live, a casebook version is published and locked from further updates. Only brand-new casebook versions can be used for design changes.
- The Clinical Data Studio application allows for export of a Study Design Specification (SDS), plus annotated workbook (PDF) - by casebook version.
- The SDS describes the entire Study design, but is not a job that can be started via the API. Further, the resulting zip file containing Excel/PDF content can be problematic for automating.
- The primary Study design format for automation needs is the Casebook Design Export (next section), a JSON based representation for the Study design.
- Studies go live - typically- on casebook version 1. Then, as changes / amendments happen, additional casebook versions are created, deployed, and Sites are activated to them, plus Subjects get migrated forward to the new design.
Casebook Design Export
Overview
- The Casebook Design Export (CDE) is a JSON representation of the Study's design, at a specific Casebook Version.
- Full JSON file for example Study : ExampleStudy-24-01_cde_v1_2024-02-16T15-51-56.json
How to Generate the CDE
To generate the CDE:
1. Determine which Casebook Versions are available for a Study: Retrieve Studies
2. Start the job against the desired Casebook Version: Start Casebook Definition Export Job.
3. Check when the job completes: Retrieve Job Status
4. When the job completes successfully, retrieve the output of the job, a JSON file: Retrieve Job Output File
Overall Structure
Overall Structure of CDE
{
"study_name": "ABCP-2023-01_DEV1",
"study_label": "ABCP-2023-01_DEV1",
"study": "ABCP-2023-01_DEV1",
"study_external_id": "ABCP-2023-01",
"name": "Initial Version",
"label": "Initial Version",
"version": 1,
"external_id": "Initial Version",
"file_name": "ABCP-2022-01_DEV1_cde_v1_2022-07-13T12-02-33.json",
"created_date": "2023-10-27T20:32:01",
"eventgroup_def": [
:
:
:
],
"form_def": [
:
:
:
],
"coding_form_def": [
:
:
:
],
"unit_def": [
:
:
:
],
"codelist_def": [
:
:
:
]
"study_setting": [
:
:
:
]
}
| Section | Description | Relevant SDS Tab(s) |
|---|---|---|
| (top) | High level information on the Study, see the table below | |
eventgroup_def |
The ordered schedule of the Study. The content will be a hierarchy of Event Group -> Event -> Form for the full schedule at the Casebook Version | Schedule - Grid, Schedule - Tree, and Repeating Event Groups |
form_def |
From Form down through Codelist / Unit Definition, how each Form is structured. A Form that appears many times in the schedule section (event_group_def) is shown once in this section |
Form Definitions |
coding_form_def |
For any Form where an Item is configured for Medical Coding, an entry appears in this section | Medical Coding Items |
unit_def |
Any unit definition in the Study schedule appears as an entry in this section. Examples - Weight, Height | Unit Codelists |
codelist_def |
Any codelist in the Study schedule appears as an entry in this section. | Codelists |
study_setting |
Studio's Study Settings page and other top level Study settings appear in this section | Study Settings |
| Path | Type | Notes |
|---|---|---|
study_name |
String | Value used in all endpoints to identify the Study. Alternatively, all endpoints will accept study (matching the label) at API release v24.2. |
study_label |
String | WARNING The study_name can be different from the label shown in the UI. |
study |
String | Always the same value as Study label, provided for clarity, as with Retrieve Studies. This will be an option on all APIs (instead of study_name) at API release v24.2. |
study_external_id |
String | Also known as 'OID' |
name |
String | |
label |
String | |
version |
Integer | |
external_id |
String | |
file_name |
String | |
created_date |
String | UTC value as YYYY-MM-DDTHH:MI:SSZ |
Schedule - Event Group Level
Schedule - Event Group Level (eventgroup_def)
:
:
"eventgroup_def": [
{
"name": "egSCR",
"label": "Screening",
"override_label": "Screening",
"external_id": "egSCR",
"description": "My description here..",
"help_content": "Some help content here..",
"repeating": false,
"repeat_maximum": null,
"type": "edc__v",
"dynamic_rule": [],
"repeat_label": [],
"event_def" : [
:
:
:
]
},
:
:
{
"name": "egCYCLES",
"label": "Repeating Cycle",
"override_label": "Repeating Cycle",
"external_id": "egCYCLES",
"description": null,
"help_content": null,
"repeating": true,
"repeat_maximum": 50,
"type": "edc__v",
"dynamic_rule": [
{
"name": "ruleENROLL_IN_STUDY",
"rule_status": "active__v",
"description": "When enrollment question is answered yet, the event group is added."
}
],
"repeat_label": [
{
"sequence": 1,
"label": "Cycle 1"
},
{
"sequence": 2,
"label": "Cycle 2"
},
{
"sequence": 2,
"label": "Cycle 2"
}
:
:
{
"sequence": 50,
"label": "Cycle 50"
}
],
"event_def" : [
:
:
:
]
},
:
:
{
"name": "egUNS",
"label": "Unscheduled",
"override_label": "Unscheduled",
"external_id": "egUNS",
"description": null,
"help_content": null,
"repeating": true,
"repeat_maximum": 10,
"type": "EDC",
"dynamic_rule": [],
"repeat_label": [],
"event_def" : [
:
:
:
]
}
]
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
eventgroup_def/ |
Tree | (Array) Ordered the same as Studio's schedule view |
eventgroup_def/name |
Tree - C | (String) |
eventgroup_def/label |
Tree - B | (String) |
eventgroup_def/override_label |
Tree - B | (String) When a design element is used more than once in a schedule, it can have different labels at the various locations |
eventgroup_def/external_id |
Tree - O | (String) Also known as 'OID' |
eventgroup_def/description |
Tree - Z | (String) |
eventgroup_def/help_content |
- | (String) Future release field, exists in Studio design, but not shown in the Study, so excluded from SDS |
eventgroup_def/repeating |
Tree - I | (Boolean) |
eventgroup_def/repeat_maximum |
Tree - J | (Integer) When the Event Group repeats, the max is allowed |
eventgroup_def/type |
Tree - M | (String) |
eventgroup_def/dynamic_rule/ |
Tree - AB | (Array) Empty array if no dynamic Rules |
eventgroup_def/dynamic_rule/name |
Tree - AB | (String) |
eventgroup_def/dynamic_rule/rule_status |
Rules - M | (String) |
eventgroup_def/dynamic_rule/description |
Rules - H | (String) |
eventgroup_def/repeat_label/ |
Tree - AA and Repeating Event Groups | (String) Optional labels for each sequence or instance when the Event Group repeats |
eventgroup_def/repeat_label/sequence |
Tree - AA | (Integer) |
eventgroup_def/repeat_label/label |
Tree - AA | (String) |
eventgroup_def/event_def/ |
- | (Array) Events of the Event Group, per the Study schedule (see later section) |
In the schedule view of Studio pictured, the Event Groups are the outermost boxes - Screening and On Study:
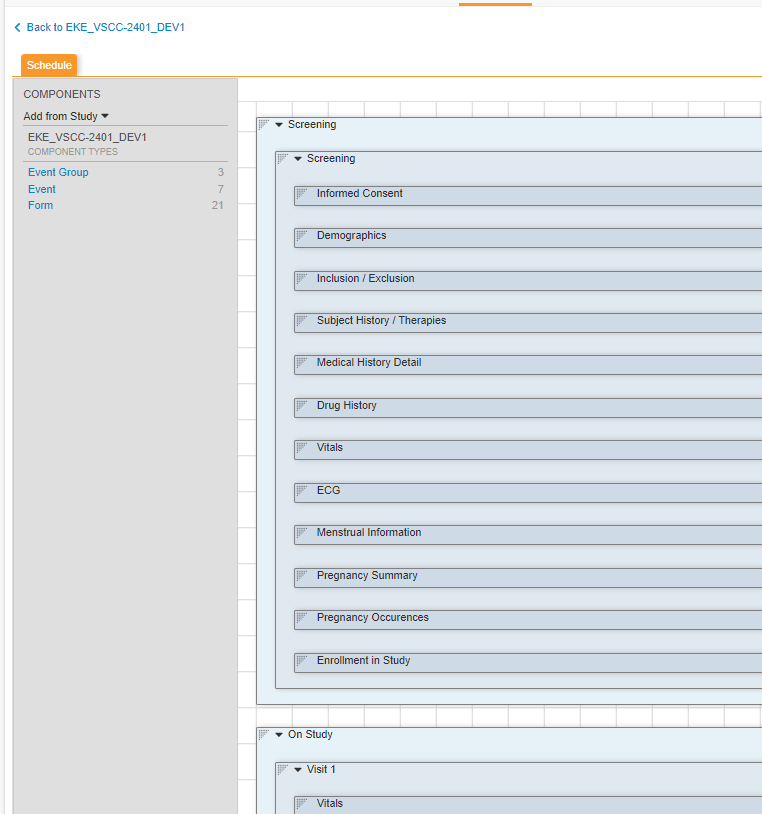
For additional information regarding Event Groups, Events, and Study schedule see Clinical Data Help
Schedule - Event Level
Schedule - Event Level (event_def)
"eventgroup_def": [
:
:
:
"event_def": [
{
"name": "evSCR",
"label": "Screening",
"override_label": "Screening",
"short_label": "SCR",
"override_short_label": "SCR",
"external_id": "evSCR",
"description": "Screening Event",
"help_content": "Subject Screening Event",
"type": "edc__v",
"dynamic": false,
"dynamic_rule": null,
"overdue_days": 10,
"open_query_future_date": false,
"open_query_out_of_window": false,
"event_window": [],
"method": [],
"form_def": [
:
:
:
]
},
{
"name": "evV1",
"label": "Visit 1",
"override_label": "Visit 1",
"short_label": "V1",
"override_short_label": "V1",
"external_id": "evV1",
"description": null,
"help_content": null,
"type": "edc__v",
"dynamic": true,
"dynamic_rule": [
{
"name": "ruleV1Add",
"rule_status": "active__v",
"description": "Visit 1 added when screening indicated as complete and enrolled"
},
{
"name": "ruleV1Add_CBOld",
"rule_status": "inactive__v",
"description": "Visit 1 added when screening indicated as complete and enrolled (previous CB versions)"
}
],
"overdue_days": 10,
"open_query_future_date": true,
"open_query_out_of_window": true,
"event_window": [
{
"default": true,
"sequence": 0,
"offset_type": "specific_event__v",
"offset_eventgroup_def": "egSCR",
"offset_event_def": "evSCR",
"offset_days": 14,
"day_range_early": 3,
"day_range_late": 3
}
],
"method": ["on_site_visit__v"],
"form_def": [
:
:
:
]
},
{
"name": "evV2",
"label": "Visit 2",
"override_label": "Visit 2",
"short_label": "V2",
"override_short_label": "V2",
"external_id": "evV2",
"description": null,
"help_content": null,
"type": "edc__v",
"dynamic": true,
"dynamic_rule": [
{
"name": "rule_continue_to_V2",
"rule_status": "active__v",
"description": "Indicating continue to V2, adds the event"
}
],
"overdue_days": 10,
"open_query_future_date": true,
"open_query_out_of_window": true,
"event_window": [
{
"default": true,
"sequence": 0,
"offset_type": "previous_event__v",
"offset_eventgroup_def": null,
"offset_event_def": null,
"offset_days": 14,
"day_range_early": 3,
"day_range_late": 3
}
],
"method": ["on_site_visit__v", "virtual_visit__v", "at_home_visit__v"],
"form_def": [
:
:
:
]
},
:
:
:
]
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
../event_def/ |
Tree | (Array) Ordered the same as Studio's schedule view |
../event_def/name |
Tree - E | (String) |
../event_def/label |
Tree - D | (String) Default label for the Event |
../event_def/override_label |
Tree - D | (String) Should the Event be used many times (different Event Groups) in the schedule, a label can be different at the various locations |
../event_def/short_label |
Tree - L | (String) Default short label for the Event |
../event_def/override_short_label |
Tree - L | (String) Should the Event be used many times (different Event Groups) in the schedule, a short label can be different at the various locations |
../event_def/external_id |
Tree - O | (String) Also known as 'OID' |
../event_def/description |
Tree - Z | (String) |
../event_def/help_content |
- | (String) Future release field, exists in Studio design, but not shown in the Study, so excluded from SDS for now |
../event_def/type |
Tree - N | (String) |
../event_def/dynamic |
Tree - AB | (Boolean) Whether the Event is to be dynamic, i.e. added by a Rule |
../event_def/dynamic_rule/ |
Tree - AB | (Array) Empty array if no dynamic Rules |
../event_def/dynamic_rule/name |
Tree - AB | (String) |
../event_def/dynamic_rule/rule_status |
Rules - M | (String) |
../event_def/dynamic_rule/description |
Rules - H | (String) |
../event_def/overdue_days |
Tree - R | (Integer) When the Event date entered plus this many days are now past, the Forms of the Event will be considered overdue |
../event_def/open_query_future_date |
Tree - Y | (Boolean) Whether or not a future date entered for the Event should generate a Query to the data entry user |
../event_def/open_query_out_of_window |
Tree - X | (String) yes__v, no__v, or inherit__v. The inherit means yes/no stems from the Study level setting. Otherwise the yes/no are 'at that Event' behavior |
../event_def/event_window |
Tree and Repeating Event Groups | (Array) Series of Event window rules, e.g. Visit 1 has a target of 7 days from Screening, with an allowed window of 2 days before or after the target |
../event_def/event_window/default |
- | (Boolean) If the Event is the first of a series of repeating in the Event Group, this will be true. Also true for non-repeating |
../event_def/event_window/sequence |
- | (Number) Will move 0,1,2.. for the sequence, when the Event is in a repeating Event Group |
../event_def/event_window/offset_type |
Tree - Q | (String) Whether the previous Event in the schedule (or sequence) is used, or a specific Event |
../event_def/event_window/offset_eventgroup_def |
Tree - R | (String) When the window is to a specific Event, the Event Group name where the related Event resides |
../event_def/event_window/offset_event_def |
Tree - R | (String) When the window is to a specific Event, that Event name |
../event_def/event_window/day_range_early |
Tree - U | (Integer) The Event date would be on/after this many days before the target to be considered 'in window' |
../event_def/event_window/offset_days |
Tree - T | (Integer) The target for the Event 'from' (in days) the related Event |
../event_def/event_window/day_range_late |
Tree - V | (Integer) The Event date would be on/before this many days after the target to be considered 'in window' |
../event_def/method |
Tree - P | (Array of String) Valid visit method values at this location in the Study schedule. The option must be Study enabled and valid choices are also configured per the needs of the Study. |
../event_def/form_def/ |
(next section) | (Array) Forms of the Event in the Study schedule (see next section) |
In the schedule view of Studio pictured, the Events are the boxes inside the outermost boxes (Event Groups) - Screening and Visit 1:

Additional information regarding Event Groups, Events, and Study schedule -> see CDMS API Help
Schedule - Form Level
Schedule - Form Level (form_def inside evengroup_def)
"eventgroup_def": [
:
:
:
"event_def": [
:
:
:
"form_def": [
{
"name": "DM",
"label": "Subject Demographics",
"override_label": "Demography",
"short_label": "Demog",
"override_short_label": "DM",
"repeating": false,
"repeat_maximum": null,
"dynamic": false,
"dynamic_rule": []
},
{
"name": "VS",
"label": "Vitals",
"override_label": "Vitals",
"short_label": "VS",
"override_short_label": "VS",
"repeating": false,
"repeat_maximum": null,
"dynamic": false,
"dynamic_rule": []
},
{
"name": "IE",
"label": "Inclusion / Exclusion Criteria",
"override_label": "Inclusion / Exclusion Criteria",
"short_label": "Inc/Exc Criteria",
"override_short_label": "Inc/Exc Criteria",
"repeating": false,
"repeat_maximum": null,
"dynamic": false,
"dynamic_rule": [],
},
{
"name": "PREG_INFO",
"label": "Pregnancy Information",
"override_label": "Pregnancy Information",
"short_label": "Preg Info",
"override_short_label": "Preg Info",
"repeating": true,
"repeat_maximum": 5,
"dynamic": true,
"dynamic_rule": [
{
"name": "rule_FEMALE_PREG_INFO_SHOW",
"rule_status": "active__v",
"description": "form is added only when gender = female"
}
]
}
:
:
:
]
]
]
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
../form_def/ |
Tree | (Array) Ordered the same as Studio's schedule view, shortened version of the Form, the next section describes 'form down' design |
../form_def/name |
Tree - G | (String) Default label for the Form |
../form_def/label |
Tree - F | (String) Should the Form be used many times (different Form) in the schedule, a label can be different at the various locations |
../form_def/override_label |
Tree - F | (String) Should the Form be used many times (different Events) in the schedule, a label can be different at the various locations |
../form_def/short_label |
Tree - L | (String) Default short label for the Form |
../form_def/override_short_label |
Tree - L | (String) Should the Form be used many times (different Events) in the schedule, a short label can be different at the various locations |
../form_def/repeating |
Tree - I | (Boolean) |
../form_def/repeat_maximum |
Tree - J | (Integer) When the Form repeats, the max allowed |
../form_def/dynamic |
Tree - AB | (Boolean) Whether the Form is to be dynamic, i.e. added by a Rule |
../form_def/dynamic_rule/ |
Tree - AB | (Array) |
../form_def/dynamic_rule/name |
Tree - AB | (String) |
../form_def/dynamic_rule/rule_status |
Rules - M | (String) |
../form_def/dynamic_rule/description |
Rules - H | (String) |
In the schedule view of Studio pictured, the Forms are those at the level of (example) Informed Consent, ECG, and Demographics:

Additional information regarding Event Groups, Events, and Study schedule -> see CDMS API Help
Form Definitions - Form Level
Form Definitions - Form Level (form_def standalone level)
:
:
"form_def": [
{
"name": "VS",
"label": "Vitals",
"short_label": "VS",
"external_id": "VS",
"special_type": [],
"description": "Vitals Form",
"help_content": "Enter Patient Vitals",
"sdtm_name": "VS",
"repeating": false,
"repeat_maximum": null,
"restricted": false,
"linking_detail": [],
"itemgroup_def": [
:
:
:
]
},
{
"name": "AE",
"label": "Adverse Events",
"short_label": "AEs",
"external_id": "AE",
"special_type": [],
"description": null,
"help_content": null,
"sdtm_name": "AE",
"repeating": true,
"repeat_maximum": 99,
"restricted": false,
"linking_detail": [
{
"remote_form_def": "CM",
"form_linked_warn_empty" true,
"description": "For SAE to CM linking"
},
{
"remote_form_def": "MH",
"form_linked_warn_empty" false,
"description": "For SAE to MH linking"
}
],
"itemgroup_def": [
:
:
:
]
},
{
"name": "CHEM",
"label": "Chemistry Panel",
"short_label": "Chemistry",
"external_id": "CHEM",
"special_type": ["lab__v"],
"description": null,
"help_content": null,
"sdtm_name": "LB",
"repeating": false,
"repeat_maximum": null,
"restricted": false,
"linking_detail": [],
"itemgroup_def": [
:
:
:
]
},
:
:
]
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
form_def/ |
Form Definitions | (Array) Unique Forms that exist somewhere in the casebook version's schedule. If the schedule (shown in eventgroup_def) shows a Form many times, it only appears once in this section |
form_def/name |
Form Definitions - A | (String) |
form_def/label |
Form Definitions - B | (String) If an override label is used on a Form that appears in several Events of the full schedule, which is noted in the schedule (eventgroup_def) section for the locations that use an override. |
form_def/short_label |
Form Definitions - B | (String) If an override short label is used on a Form that appears in several Events of the full schedule, which is noted in the schedule (eventgroup_def) for the locations that use an override. |
form_def/external_id |
Form Definitions - P | (String) Also known as 'OID' |
form_def/special_type |
- | (Array of Strings) Values only appear if the Form behaves differently than standard EDC Forms. For example, a Local Labs Form will indicate lab__v as a special type |
form_def/description |
Form Definitions - AS | (String) |
form_def/help_content |
- | (String) Future release field, exists in Studio design, but not shown in the Study, so excluded from SDS for now |
form_def/sdtm_name |
Form Definitions - AV | (String) Property - optional - regarding data exports and SDTM format |
form_def/repeating |
Tree - I | (Boolean) |
form_def/repeat_maximum |
Tree - J | (Integer) When the Form repeats, the max allowed |
form_def/restricted |
Tree - H | (Boolean) Whether or not the Form is specially protected from certain roles in the Study (UI, reports, exports) |
form_def/linking_detail/ |
Form Definitions - E | (Array) Each is a linking definition for a pair of Forms. At either Form the end user can do Form to Form Linking. Item to Form Linking information is found at the item_def level. |
form_def/linking_detail/remote_form_def |
- | (String) Remote Form that can be linked to this Form |
form_def/linking_detail/form_linked_warn_empty |
- | (Boolean) Whether or not to warn an end user if no links are added |
form_def/linking_detail/description |
- | (String) |
form_def/itemgroup_def/ |
(next section) | (Array) Item Groups of the Form (see next section) |
In the Studio view pictured, the Demographics Form content (its Item Groups and Items) is shown:
Form Definitions - Item Group Level
Form Definitions - Item Group Level (itemgroup_def)
:
:
"form_def": [
{
"name": "VS",
:
:
:
"itemgroup_def": [
{
"name": "igSUMMARY_VS",
"label": "Vitals Summary",
"override_label": "Summary",
"external_id": "igSUMMARY_VS",
"description": null,
"help_content": null,
"special_type": [],
"sdtm_name": "VS",
"repeating": false,
"repeat_maximum": null,
"display_format": null,
"header_visible": true,
"visual_group": true,
"progressive_display": null,
"item_def": [
:
:
:
]
},
{
"name": "igREP_VS",
"label": "Vitals Taken",
"override_label": "Vitals",
"external_id": "igREP_VS",
"description": null,
"help_content": null,
"special_type": [],
"sdtm_name": "VS",
"repeating": true,
"repeat_maximum": 5,
"display_format": "editable_grid__v",
"header_visible": true,
"visual_group": true,
"progressive_display": {
"controlling_itemgroup_def": "igSUMMARY_VS",
"controlling_item_def": "VS_YN",
"values": ["Y"],
"display_type_enable": true
},
"item_def": [
:
:
:
]
}
]
},
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
../itemgroup_def/ |
Form Definitions | (Array) Ordered the same as Studio's schedule view, the Item Groups of the Form in the casebook version. |
../itemgroup_def/name |
Form Definitions - G | (String) |
../itemgroup_def/label |
Form Definitions - H | (String) |
../itemgroup_def/override_label |
Form Definitions - T | (String) When an Item Group is used in different Forms of the schedule, it can have different labels at the various locations |
../itemgroup_def/external_id |
Form Definitions - P | (String) Also known as 'OID' |
../itemgroup_def/description |
Form Descriptions - AS | (String) |
../itemgroup_def/help_content |
- | (String) Future release field, exists in Studio design, but not shown in the Study, so excluded from SDS for now |
../itemgroup_def/special_type |
Form Definitions - AQ | (Array of String) Picklist names - lab__v, lab_header__v - special types for the Item Group |
../itemgroup_def/sdtm_name |
Form Definitions - AV | (String) Property - optional - regarding data exports and SDTM format |
../itemgroup_def/repeating |
Form Definitions - I | (Boolean) |
../itemgroup_def/repeat_maximum |
Form Definitions - I | (Integer) When the Item Group repeats, the max allowed |
../itemgroup_def/display format |
Form Definitions - M | (String) When the Item Group repeats, how it is displayed on the Form. Values - form__v, read_only_grid__v, editable_grid__v |
../itemgroup_def/header_visible |
Form Definitions - J | (Boolean) |
../itemgroup_def/visual_group |
Form Definitions - K | (Boolean) |
../../progressive_display |
Form Definitions | (JSON Section) When progressive display involved in the UI, the Item and it's value that drive this Item Group visually. (show/hide or enable/disable style is used) |
../../progressive_display/controlling_itemgroup_def |
Form Definitions - W | (String) The Item Group on the Form of the Item whose value(s) dictates the progressive display |
../../progressive_display/controlling_item_def |
Form Definitions - W | (String) The other Item on the Form it's value(s) that dictates the progressive display |
../../progressive_display/values |
Form Definitions - X | (String) Values of that Item that dictate the progressive display |
../../progressive_display/display_type_enable |
Form Definitions - Y | (Boolean) Whether the style of progressive display is enable/disable or not. (false means show/hide style is instead used) |
In the Studio view pictured, General and Race/Ethnicity are Item Groups of the Form Demographics:

Form Definitions - Item Level
Form Definitions - Item Level (Date Only)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDM",
:
:
"item_def": [
{
"name": "DOB",
"label": "Date of Birth",
"override_label": null,
"short_label": "DOB",
"override_short_label": null,
"hint_label": "For entry of only birth year, use unknown month and day",
"external_id": "DOB",
"description": null,
"help_content": null,
"sdtm_name": "SEX",
"item_type": ["edc__v"],
"data_type": "date__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": true,
"query_for_ilb": true,
"query_for_future_date": true,
"query_range_minimum": "2001-01-01",
"query_range_maximum": null,
"allow_unknown_day": true,
"allow_unknown_month": true,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Date Time)
:
:
"form_def": [
{
"name": "PK",
:
:
"itemgroup_def": [
{
"name": "igPK",
:
:
"item_def": [
{
"name": "PK_DATETIME",
"label": "PK Draw Date/Time (Pre Dose)",
"override_label": null,
"short_label": "Draw DT (Pre Dose)",
"override_short_label": null,
"hint_label": null,
"external_id": "PK_DATETIME",
"description": null,
"help_content": null,
"sdtm_name": "LB_PK_DT",
"item_type": ["edc__v"],
"data_type": "datetime__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": true,
"query_for_ilb": true,
"query_for_future_date": true,
"query_range_minimum": "2001-01-01",
"query_range_maximum": null,
"allow_unknown_day": true,
"allow_unknown_month": true,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Time Only)
:
:
"form_def": [
{
"name": "PK",
:
:
"itemgroup_def": [
{
"name": "igPK",
:
:
"item_def": [
{
"name": "PK_DRAW_TIME",
"label": "Time of Draw",
"override_label": null,
"short_label": "Time of Draw",
"override_short_label": null,
"hint_label": null,
"external_id": "PK_DRAW_TIME",
"description": null,
"help_content": null,
"sdtm_name": null,
"item_type": ["edc__v"],
"data_type": "time__v",
"label_type": null,
"length": 3,
"precision": 0,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Numeric without Precision)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDM",
:
:
"item_def": [
{
"name": "AGE_SCR",
"label": "Age at Screening",
"override_label": null,
"short_label": "Numeric Result",
"override_short_label": null,
"hint_label": "(years)",
"external_id": "AGE_SCR",
"description": null,
"help_content": null,
"sdtm_name": "DM_AGE",
"item_type": ["edc__v"],
"data_type": "integer__v",
"label_type": null,
"length": 3,
"precision": 0,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Numeric with Precision)
:
:
"form_def": [
{
"name": "LB",
:
:
"itemgroup_def": [
{
"name": "igLBRES",
:
:
"item_def": [
{
"name": "LB_RESULT",
"label": "Lab Result (Numeric)",
"override_label": null,
"short_label": "Numeric Result",
"override_short_label": null,
"hint_label": "When value not numeric, use the text result below",
"external_id": "LB_RESULT",
"description": null,
"help_content": null,
"sdtm_name": "BMI",
"item_type": ["edc__v"],
"data_type": "float__v",
"label_type": null,
"length": 9,
"precision": 4,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Label - non data collecting)
:
:
"form_def": [
{
"name": "LB",
:
:
"itemgroup_def": [
{
"name": "igLBRES",
:
:
"item_def": [
:
:
{
"name": "LBL_RACE",
"label": "For race, check all that apply:",
"override_label": null,
"short_label": "Check all that apply:",
"override_short_label": null,
"hint_label": null,
"external_id": "LBL_RACE",
"description": null,
"help_content": null,
"sdtm_name": null,
"item_type": ["edc__v"],
"data_type": "text__v",
"label_type": "informational__v",
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Checkbox)
:
:
"form_def": [
{
"name": "AE",
:
:
"itemgroup_def": [
{
"name": "igAEGEN",
:
:
"item_def": [
:
:
{
"name": "AESI",
"label": "Check if the event is an AESI",
"override_label": null,
"short_label": "Is AESI",
"override_short_label": null,
"hint_label": null,
"external_id": "AESI",
"description": null,
"help_content": null,
"sdtm_name": "AESI",
"item_type": ["edc__v"],
"data_type": "boolean__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Item to Form Linking)
:
:
"form_def": [
{
"name": "MH",
:
:
"itemgroup_def": [
{
"name": "igMHGEN",
:
:
"item_def": [
:
:
{
"name": "MH_REL",
"label": "Medical History Related",
"override_label": null,
"short_label": "MH Related",
"override_short_label": null,
"hint_label": null,
"external_id": "MH_REL",
"description": null,
"help_content": null,
"sdtm_name": "MH_REL",
"item_type": ["edc__v", "reference__v"],
"data_type": "text__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": {
"form_name": "MH",
"display_item_def": [
{
"itemgroup_def": "igMH_DETAILS",
"item_def": "MH_REPORTED"
},
{
"itemgroup_def": "igMH_DETAILS",
"item_def": "MHSTDT"
},
{
"itemgroup_def": "igMH_DETAILS",
"item_def": "MHENDT"
}
]
},
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Codelist)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDM",
:
:
"item_def": [
:
:
{
"name": "SEX",
"label": "Gender",
"override_label": null,
"short_label": "Gender",
"override_short_label": null,
"hint_label": null,
"external_id": "SEX",
"description": null,
"help_content": null,
"sdtm_name": "SEX",
"item_type": ["edc__v"],
"data_type": "text__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": "cl_GENDER",
"codelist_display_type": "picklist__v",
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": true,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Unit Definition)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDM",
:
:
"item_def": [
:
:
{
"name": "WEIGHT",
"label": "Weight",
"override_label": null,
"short_label": "Weight",
"override_short_label": null,
"hint_label": null,
"external_id": "WEIGHT",
"description": null,
"help_content": null,
"sdtm_name": "WEIGHT",
"item_type": ["edc__v"],
"data_type": "float__v",
"label_type": null,
"length": 4,
"precision": 1,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": "WEIGHT_UNIT",
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": true,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": "50.5",
"query_range_maximum": "90.5",
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
}
:
:
Form Definitions - Item Level (Local Lab / Analyte)
:
:
"form_def": [
{
"name": "LB",
:
:
"itemgroup_def": [
{
"name": "igLB_TEST",
:
:
"item_def": [
:
:
{
"name": "LBORRES_WBC",
"label": "White Blood Cell Count",
"override_label": null,
"short_label": "WBC",
"override_short_label": null,
"hint_label": null,
"external_id": "LBORRES_WBC",
"description": null,
"help_content": null,
"sdtm_name": "LBORRES_WBC",
"item_type": ["edc__v"],
"data_type": "float__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": "CHEM_UNITS",
"analyte_def": "wbc__v",
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": true,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
},
:
:
Form Definitions - Item Level (Involves Defaulting of Item Groups)
:
:
"form_def": [
{
"name": "PK",
:
:
"itemgroup_def": [
{
"name": "igPK",
:
:
"item_def": [
:
:
{
"name": "PK_DRAW_TIME",
"label": "Draw",
"override_label": null,
"short_label": "Draw",
"override_short_label": null,
"hint_label": null,
"external_id": "PK_DRAW_TIME",
"description": null,
"help_content": null,
"sdtm_name": "PK_TIME_PERIOD",
"item_type": ["edc__v", "read_only__v"],
"data_type": "text__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": "PK_DRAW_PERIODS",
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": null,
"default_display": [
{
"sequence": 1,
"code": "PRE_DOSE"
},
{
"sequence": 2,
"code": "HOUR_1"
},
{
"sequence": 3,
"code": "HOUR_2"
},
{
"sequence": 4,
"code": "HOUR_4"
},
{
"sequence": 5,
"code": "HOUR_8"
}
],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
}
:
:
Form Definitions - Item Level (Involves Progressive Display)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDMGEN",
:
:
"item_def": [
:
:
{
"name": "LAST_MENST",
"label": "Last Menstrual Date",
"override_label": null,
"short_label": "Last Menstrual",
"override_short_label": null,
"hint_label": null,
"external_id": "LAST_MENST",
"description": null,
"help_content": null,
"sdtm_name": "LAST_MENST",
"item_type": ["edc__v"],
"data_type": "date__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display":
{
"controlling_itemgroup": "igDEMOG_GEN",
"controlling_item": "GENDER",
"values": ["F"],
"display_type_enable": true
},
"query_required": true,
"query_for_ilb": true,
"query_for_future_date": true,
"query_range_minimum": "2023-06-30",
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
}
:
:
Form Definitions - Item Level (Derived / Read Only)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDMGEN",
:
:
"item_def": [
:
:
{
"name": "BMI",
"label": "BMI (Calculated)",
"override_label": null,
"short_label": "Time of Draw",
"override_short_label": null,
"hint_label": "This value is calculated on submit of the form",
"external_id": "BMI",
"description": null,
"help_content": null,
"sdtm_name": "BMI",
"item_type": ["edc__v", "derived__v", "read_only__v"],
"data_type": "float__v",
"label_type": null,
"length": 4,
"precision": 2,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
}
:
:
Form Definitions - Item Level (URI)
:
:
"form_def": [
{
"name": "DM",
:
:
"itemgroup_def": [
{
"name": "igDMGEN",
:
:
"item_def": [
:
:
{
"name": "MY_URL",
"label": "Enter URL of the Submitted Report",
"override_label": null,
"short_label": "URL for Report",
"override_short_label": null,
"hint_label": null,
"external_id": "MY_URL",
"description": null,
"help_content": null,
"sdtm_name": "CUS_URL",
"item_type": ["edc__v"],
"data_type": "uri__v",
"label_type": null,
"length": null,
"precision": null,
"codelist_def": null,
"codelist_display_type": null,
"indent_level": 0,
"unit_def": null,
"analyte_def": null,
"linking_detail": [],
"default_display": [],
"progressive_display": null,
"query_required": false,
"query_for_ilb": false,
"query_for_future_date": false,
"query_range_minimum": null,
"query_range_maximum": null,
"allow_unknown_day": false,
"allow_unknown_month": false,
"allow_unknown_time": false
}
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
../item_def/ |
Form Definitions | (Array) Ordered the same as Studio's schedule view, the Items in the_Item Group_ at the casebook version. |
../item_def/name |
Form Definitions - O | (String) |
../item_def/label |
Form Definitions - Q | (String) |
../item_def/override_label |
Form Definitions - T | (String) Should the Item be used many times (different Item Groups) in the schedule, a label can be different at the various locations |
../item_def/short_label |
Form Definitions - AL | (String) |
../item_def/override_short_label |
Form Definitions - AL | (String) Should the Item be used many times (different Item Groups) in the schedule, a short label can be different at the various locations |
../item_def/hint_label |
Form Definitions - AG | (String) Text to the right of the field in italics |
../item_def/external_id |
Form Definitions - P | (String) Also known as 'OID' |
../item_def/description |
Form Definitions - AS | (String) |
../item_def/help_content |
Form Definitions - AH | (String) Hover text on the Item label. In SDS, column name is Hover Help |
../item_def/sdtm_name |
Form Definitions - AV | (String) Property - optional - regarding data exports and SDTM format |
../item_def/item_type |
Form Definitions - AM to AR | (Array of String) Values in the array are the signal of the 'Yes' in the SDS column(s) |
../item_def/data_type |
Form Definitions - R | (String) |
../item_def/label_type |
Form Definitions - AR | (String) |
../item_def/length |
Form Definitions - AD | (Integer) Maximum number of digits for the whole part of a numeric value, or maximum characters for a text value |
../item_def/precision |
Form Definitions - AE | (Integer) Maximum number of digits for the decimal part of a numeric value |
../item_def/codelist_def |
Form Definitions - Z | (String) |
../item_def/codelist_display_type |
Form Definitions - AA | (String) Style of the codelist, whether Picklist, Radio Buttons (horizontal), or Radio Buttons (vertical) |
../item_def/indent_level |
Form Definitions - U | (Integer) The indent level from the left side (0 = not indented) |
../item_def/unit_def |
Form Definitions - | (String) |
../item_def/analyte_def |
Form Definitions - V | (String) If an Item that collects values for the Local Labs module, which analyte |
../item_def/linking_detail/ |
Form Definitions - AT | (JSON Section)If the Item is an Item to Form type, this is the detail of that remote Form being linked |
../item_def/linking_detail/remote_form_def |
Form Definitions - AT | (String) Remote Form being linked to by the Item |
../item_def/linking_detail/display_item_def/ |
Form Definitions - AU | (Array) Items from the Remote Form (values on it) to display inline on this Form |
../item_def/linking_detail/display_item_def/itemgroup_def |
Form Definitions - AU | (String) Item Group of the Item on the remote Form (its value) to display inline |
../item_def/linking_detail/display_item_def/item_def |
Form Definitions - AU | (String) Item on the remote Form (its value) to display inline |
../item_def/default_display/ |
Form Definitions - N | (Array) When the Item Group of the Item is involved in defaulting on first visiting the Form, these are the values seeded |
../item_def/default_display/sequence |
Form Definitions - N | (Integer) The Item Group sequence getting that value on default add |
../item_def/default_display/code |
Form Definitions - N | (Integer) The Item value being set on default add (at the sequence) |
../../progressive_display |
Form Definitions | (JSON Section) When progressive display involved in the UI, the Item and it's value that drive this Item visually. (show/hide or enable/disable style is used) |
../../progressive_display/controlling_itemgroup_def |
Form Definitions - W | (String) The Item Group on the Form of the Item whose value(s) dictates the progressive display |
../../progressive_display/controlling_item_def |
Form Definitions - W | (String) The other Item on the Form it's value(s) that dictates the progressive display |
../../progressive_display/values |
Form Definitions - X | (String) Values of that Item that dictate the progressive display |
../../progressive_display/display_type_enable |
Form Definitions - Y | (Boolean) Whether the style of progressive display is enable/disable or not. (false means show/hide style is instead used) |
../item_def/query_required |
Form Definitions - | (Boolean) Whether or not the Item requires a value. (true = query fires on the Item) |
../item_def/query_for_ilb |
Form Definitions - | (Boolean) Whether or not the Item should or should not allow the Intentionally Left Blank action. (true = query fires on the Item when set ILB) |
../item_def/query_for_future_date |
Form Definitions - | (Boolean) Whether or not the Item should allow future dates (true = query fires on the Item when value is in future) |
../item_def/query_range_minimum |
Form Definitions - | (String) Minimum value such that a value below/before (date or numeric Items) will fire a query |
../item_def/query_range_maximum |
Form Definitions - | (String) Maximum value such that a value above/after (date or numeric Items) will fire a query |
../item_def/allow_unknown_day |
Form Definitions - AF | (Boolean) For date or datetime Items, whether the day portion of the value is allowed to be unknown. (dd* will appear in SDS) |
../item_def/allow_unknown_month |
Form Definitions - AF | (Boolean) For date or datetime Items, whether the month portion of the value is allowed to be unknown. (MMM* will appear in SDS) |
../item_def/allow_unknown_time |
Form Definitions - AF | (Boolean) For datetime Items, whether the time portion of the value is allowed to be unknown. |
In the Studio view pictured, Item examples include Year of Birth, Gender, and Ethnicity:

Coding Form Definitions
Coding Form Definitions
:
:
"coding_form_def": [
{
"form_def": "AE",
"form_type": "ae__v",
"form_type_other_label": null,
"form_coding_status", "active__v",
"dictionary": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"coding_definition_name": "VV-12345",
"coding_definition_id": "XYZ000000034",
"coding_status": "active__v",
"coding_item_def": "AETERM",
"related_item_def": [ ]
},
{
"form_def": "CM",
"form_type": "conmed__v",
"form_type_other_label": null,
"form_coding_status", "active__v",
"dictionary": "WHODrug",
"dictionary_release": "GLOBALC3Mar23",
"coding_definition_name": "VV-12347",
"coding_definition_id": "XYZ000000037",
"coding_status": "needs_dictionary__v",
"coding_item_def": "CMTERM",
"related_item_def": [
{
"item_def": "CMDOSE",
"type": "dose__v",
"type_other": null
},
{
"item_def": "CMROUTE",
"type": "route__v",
"type_other": null
}
]
},
{
"form_def": "DTH",
"form_type": "other__v",
"form_type_other_label": "Death Cause",
"form_coding_status", "active__v",
"dictionary": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"coding_definition_name": "VV-12349",
"coding_definition_id": "XYZ000000040",
"coding_status": "active__v",
"coding_item_def": "REP_CAUSE",
"related_item_def": [
{
"item_def": "CAUSE_TYPE",
"type": "other__v",
"type_other": "Death Cause Type"
}
]
}
]
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
coding_form_def/ |
Medical Coding Items | (Array) Each Form in the Study that is configured for medical coding. See also Retrieve Coding Definitions |
coding_form_def/form_def |
Medical Coding Items - A | (String) The Form design name where coding happens |
coding_form_def/form_type |
Medical Coding Items - C | (String) Type of coding taking place on the Form |
coding_form_def/form_type_other_label |
Medical Coding Items - C | (String) When form_type = other__v, the label to use in in parenthesis in the SDS |
coding_form_def/form_coding_status |
- | (String) |
coding_form_def/dictionary |
- | (String) |
coding_form_def/dictionary_release |
- | (String) |
coding_form_def/coding_definition_name |
- | (String) The Vault internal name__v of the definition |
coding_form_def/coding_definition_id |
- | (String) The Vault internal id of the definition |
coding_form_def/coding_status |
- | (String) |
coding_form_def/coding_item_def |
Medical Coding Items - E | (String) The Item on the Form whose value will take part in medical coding |
coding_form_def/related_item_def/ |
Medical Coding Items | (Array) Other Items on the Form (their values) that will be stored on the coding request |
coding_form_def/related_item_def/item_def |
Medical Coding Items - G | (String) |
coding_form_def/related_item_def/type |
Medical Coding Items - I | (String) |
coding_form_def/related_item_def/type_other |
Medical Coding Items - I | (String) |
In the Studio view pictured, the Form ConMed has a Drug Name Item being coded, with additional properties for the coding request from other Items on the same Form:
Codelists
Codelists
:
:
"codelist_def": [
{
"name": "cYN",
"external_id": "cYN",
"description": "My description text here",
"lab_related": false,
"choice": [
{
"sequence": 1,
"code": "Y",
"label": "Yes",
"description": null,
"hidden": false
},
{
"sequence": 2,
"code": "N",
"label": "No",
"description": null,
"hidden": false
}
]
},
{
"name": "AEOUT",
"external_id": "AEOUT",
"description": null,
"lab_related": false,
"choice": [
{
"sequence": 1,
"code": "RECOVERED",
"label": "Recovered / Resolved",
"description": null,
"hidden": false
},
{
"sequence": 2,
"code": "RECOVERING",
"label": "Recovering / Resolving",
"description": null,
"hidden": false
},
{
"sequence": 3,
"code": "NOT_RECOVERED",
"label": "Not Recovered / Resolved",
"description": null,
"hidden": false
},
{
"sequence": 4,
"code": "RECOVERED_W_SEQ",
"label": "Recovered / Resolved with Sequelae",
"description": null,
"hidden": false
},
{
"sequence": 5,
"code": "FATAL",
"label": "Fatal",
"description": null,
"hidden": false
},
{
"sequence": 6,
"code": "UNKNOWN",
"label": "Unknown",
"description": null,
"hidden": false
}
]
},
:
:
:
]
:
:
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
codelist_def/ |
Codelists | (Array) Each Codelist that appears at least once in an Item of the casebook version |
codelist_def/name |
Codelists - A | (String) |
codelist_def/external_id |
Codelists - B | (String) |
codelist_def/description |
Codelists - C | (String) |
codelist_def/lab_related |
Codelists - H | (Boolean) Whether or not the Codelist is part of the Local Labs module |
codelist_def/choice/ |
Codelists | (Array) The choices of the Codelist |
codelist_def/choice/sequence |
Codelists | (Integer) Order is 'reading down' in Studio, SDS, EDC UI |
codelist_def/choice/code |
Codelists - E | (String) |
codelist_def/choice/label |
Codelists - F | (String) |
codelist_def/choice/description |
Codelists - C | (String) |
codelist_def/choice/hidden |
Codelists - G | (Boolean) Whether the choice is hidden or not, in the casebook version |
Unit Definitions
Unit Definitions
:
:
"unit_def": [
{
"name": "uclWEIGHT",
"label": "Weight - KG / LB",
"external_id": "uclWEIGHT",
"description": "My description text here",
"lab_related": false,
"choice": [
{
"sequence": 1,
"name": "kg",
"label": "Kilograms",
"description": null,
"hidden": false,
"standard": true,
"abbreviation": "KG",
"external_id": "KG",
"unit_conversion": null
},
{
"sequence": 2,
"name": "lbs",
"label": "Pounds",
"description": null,
"hidden": false,
"standard": false,
"abbreviation": "LBS",
"external_id": "LBS",
"unit_conversion": "$value__v /2.2046"
}
]
},
:
:
]
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
unit_def/ |
Unit Codelists | (Array) Each Unit Definition that appears at least once in an Item of the casebook version |
unit_def/name |
Unit Codelists - A | (String) |
unit_def/label |
- | (String) |
unit_def/external_id |
Unit Codelists - H | (String) |
unit_def/type |
Unit Codelists - C | (String) |
unit_def/description |
Unit Codelists - B | (String) |
unit_def/lab_related |
Unit Codelists - J | (Boolean) Whether or not the Unit Definition is part of the Local Labs module |
unit_def/choice/ |
Unit Codelists | (Array) The choices of the Unit |
unit_def/choice/sequence |
Unit Codelists | (Integer) Order is 'reading down' in Studio, SDS, EDC UI |
unit_def/choice/name |
Unit Codelists - A | (String) |
unit_def/choice/code |
Unit Codelists - D | (String) |
unit_def/choice/label |
Unit Codelists - E | (String) |
unit_def/choice/description |
Unit Codelists - B | (String) |
unit_def/choice/hidden |
Unit Codelists - I | (Boolean) Whether the choice is hidden or not, in the casebook version |
unit_def/choice/standard |
Unit Codelists - F | (Boolean) Whether or not the unit choice is the Study default unit |
unit_def/choice/abbreviation |
- | (String) |
unit_def/choice/external_id |
Unit Codelists - H | (String) |
unit_def/choice/unit_conversion |
Unit Codelists - G | (String) The formula used to convert a value of that unit to the default unit |
Additional information regarding Unit Definitions -> see CDMS API Help
Study Settings
Study Settings
:
:
"study_setting": [
{
"setting_name": "environment_type",
"setting label": "Environment Type",
"value": "development__v",
"value_label": "Development"
},
{
"setting_name": "study_phase",
"setting label": "Study Phase",
"value": "phase_i__v",
"value_label": "Phase I"
},
{
"setting_name": "standard_date_format",
"setting_label": "Standard Date Format",
"value": "dd-MMM-yyyy"
},
:
:
]
| Path | SDS Tab - Column | Type / Notes |
|---|---|---|
study_setting/ |
Study Settings | (Array) |
study_setting/setting_name |
Study Settings | (String) |
study_setting/setting_label |
Study Settings | (String) |
study_setting/value |
Study Settings | (String) |
study_setting/value_label |
Study Settings | (String) When the value originates from a Vault Picklist, then this is the picklist label. (value = picklist name). This property is omitted from the entry when the setting is not of picklist type |
Additional information regarding Study settings:
| Setting Name | Setting Label | Location / Notes |
|---|---|---|
environment_type |
Environment Type | Picklist, so both value and value_label to be shown |
study_instance_label |
Study Label | |
external_id |
External ID | (Studio) |
study_phase |
Study Phase | (Studio) Picklist, so both value and value_label to be shown |
study_status |
CDMS Study Status | (Studio) Picklist, so both value and value_label to be shown |
standard_date_format |
Standard Date Format | (Studio) |
twelve_hour_time |
Twelve Hour Time | (Studio) Values: true/ false |
reason_for_change_other_specify |
Enable Other Specify - Reason for change | (Studio) Values: true/ false |
intentionally_left_blank_other_specify |
Enable Other Specify - Intentionally Left Blank | (Studio) Values: true/ false |
enable_team_query_restrictions |
Enable Team Query Restrictions | (Studio) Values: true/ false |
system_query_team |
Query Team for System Queries | (Studio) Values: (plain text, field is Text(50)) |
enable_randomization |
Enable Veeva Randomization | |
enable_local_labs |
Enable Local Labs | (Studio) Values: true/ false |
safety_integration_type |
Safety Integration Type | (Studio) Picklist, so included value AND value_label in return |
lab_default_fire_queries |
Local Labs - Fire Out-of-range Queries by Default | (Labs) Omitted when enable_local_labs = false |
age_calculations_default_values |
Local Labs - Default Day and Month for Age Calculations (MM-DD) | (Labs) Omitted when enable_local_labs = false |
lab_clinical_significance |
Local Labs - Enable Clinical Significance | (Labs) Omitted when enable_local_labs = false |
enable_normal_override |
Local Labs - Enable Normal Override | (Labs) Omitted when enable_local_labs = false |
disable_unit_selection_for_lab_results |
Local Labs - Disable Unit Selection for Results | (Labs) Omitted when enable_local_labs = false |
enable_protocol_deviations |
Enable Protocol Deviations | (Studio) Values: true/ false |
enable_additive_review |
Enable Additive Review | (Studio) Values: true/ false |
subject_id_gen_type |
Subject ID Generation - Type | (Studio) Picklist, so both value and value_label to be shown |
subject_id_gen_format |
Subject ID Generation - Format | (Studio) |
subject_id_range_start |
Subject ID Generation - Range Start | (Studio) |
subject_id_range_end |
Subject ID Generation - Range End | (Studio) |
event_out_of_window_add_query |
Event Out of Window Add Query | (Studio) Values: true/ false |
enable_assessments |
Enable Assessments | (Studio) Values: true/ false |
Retrieve Casebook Definition Versions
Request - Retrieve Studies (for Casebook Versions)
curl -L -X GET 'https://myvault.veevavault.com/api/v24.1/app/cdm/studies' \
-H 'Accept: application/json' \
-H 'Authorization: {SESSION_ID}'
Response - Retrieve Studies (for Casebook Versions)
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 2,
"total": 2
},
"studies": [
{
"study_name": "Labrinone_DEV1",
"external_id": "Labrinone",
"study_phase": "Phase III",
"study_status": "Execution",
"casebook_versions": [
{
"study_name": "Labrinone_DEV1",
"casebook_version": 1,
"version_name": "Initial Version",
"external_id": "1",
"previous_version_name": null,
"description": null,
"change_reason": null,
"casebook_status": "in_progress__v",
"created_by": "Eric Emerton",
"created_date": "2018-02-12T22:32:21Z",
"last_modified_date": "2020-03-04T14:16:20Z"
}
]
},
{
"study_name": "ABCP-2022-01_DEV1",
"external_id": "ABCP-2022-01",
"study_phase": "Phase I",
"study_status": "Planning",
"casebook_versions": [
{
"study_name": "ABCP-2022-01_DEV1",
"casebook_version": 2,
"version_name": "Version2",
"external_id": "Initial Version",
"previous_version_name": null,
"description": "Casebook Definition for the ABCP-2022-01_DEV1 study" ,
"change_reason": "Layout changes",
"casebook_status": "in_progress__v",
"created_by": "Maisha Muddaththir",
"created_date": "2023-06-09T13:26:34Z",
"last_modified_date": "2023-06-09T13:26:34Z"
},
{
"study_name": "ABCP-2022-01_DEV1",
"casebook_version": 1,
"version_name": "Initial Version",
"external_id": "Initial Version",
"previous_version_name": null,
"description": "Initial casebook definition version for the ABCP-2022-01_DEV1 study" ,
"change_reason": null,
"casebook_status": "published__v",
"created_by": "Maisha Muddaththir",
"created_date": "2023-06-09T13:26:34Z",
"last_modified_date": "2023-06-09T13:26:34Z"
}
]
}
]
}
Currently, the casebook definitions are available via the Retrieve Studies API. The return comprised what casebook versions exist, but not the schedule / detail of each.
Retrieve Coding Definitions
Request
curl -L -X GET 'https://myvault.veevavault.com/api/v24.1/app/cdm/coder/forms?study_name=ABCP-2022-01_DEV1' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 3,
"total": 3
},
"forms": [
{
"form_name": "AE",
"form_type": "ae__v",
"form_definition_status": "active__v",
"form_coding_status": "active__v",
"dictionary_definition": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"coding_item_definition_id": "V0T000000002001",
"coding_item_definition_name": "VV-000004"
},
{
"form_name": "MULTI_CODING",
"form_type": "other__v",
"form_type_other_label": "My Other Label Here",
"form_definition_status": "active__v",
"form_coding_status": "active__v",
"dictionary_definition": "MedDRA",
"dictionary_release": "MedDRA_25_1",
"coding_item_definition_id": "V0T000000003001",
"coding_item_definition_name": "VV-000005"
},
{
"form_name": "Concomitant-Medication",
"form_type": "conmed__v",
"form_definition_status": "active__v",
"form_coding_status": "active__v",
"dictionary_definition": "WHODrug C3",
"dictionary_release": "GLOBALC3Mar22",
"coding_item_definition_id": "V0T000000004001",
"coding_item_definition_name": "VV-000006"
}
]
}
Notes
- Retrieves a list of coding-enabled Form Definitions in a Study.
- This API returns values required to use the Retrieve Coding Requests endpoint.
- Depending on Study design changes, additional entries can/will be returned, but with
inactive__vvalue forform_definition_status. These additional entries might have older coding requests tied to them, but the Form has since updated to a new coding configuration, it tied to the newer coding requests
Endpoint
GET /api/{version}/app/cdm/coder/forms
Required Permissions
The following permissions are required to use the Retrieve Coding Definitions API:
- API Access
- View Code
The CDMS API Read Only and CDMS API Read Write roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query Parameters
Include the following parameters to filter the results:
| Name | Description |
|---|---|
study_name |
The Name (Study Number) of the Study (study__v.name__v) that you want to retrieve the coding-enabled Form Definitions for, returned as study_name by the Retrieve Studies API. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer). If omitted, the default is 1. For example, if the limit is set to "100", and there are 150 possible records, use "101" as the offset to show results 101 through 150. |
Response Details
On SUCCESS, Vault returns a list of Forms (as JSON array entries) where Medical Coding is configured to occur. The information includes:
| Array | Name | Description |
|---|---|---|
forms |
form_name |
Study design name for a Form where Medical Coding is configured to occur. |
forms |
form_type |
The picklist name of the type of coding - ae__v, conmed__v, medical_history__v, or other__v. |
forms |
form_definition_status |
The picklist name for status of the definition. Possible values are active__v or inactive__v. |
forms |
form_coding_status |
The picklist name for current status within the Study. Possible values are active__v (creating coding requests allowed), needs_dictionary__v (no dictionary version set yet), needs_synonym_list__v, study_locked__v, upversioning__v, or failed_upversioning__v. |
forms |
dictionary_definition |
The name value from the Vault's configured Medical Coding Dictionary Definition records. These values are typically one of MedDRA, WHODrug B3, WHODrug C3, or Custom. |
forms |
dictionary_release |
Name value of the dictionary in use at the coding location. Examples - MedDRA_25_1, GLOBALC3Mar22 |
forms |
coding_item_definition_id |
The Vault internal ID of the Medical Coding Item Definition. This value is used in Retrieve Coding Requests. |
forms |
coding_item_definition_name |
The Vault name value of the Medical Coding Item Definition, for additional reference. Previously this was used in retrieving specific Medical Coding Requests. |
Retrieve Study Masters
Request - Get study masters in a vault
curl -L -X GET 'https://myvault.veevavault.com/api/v24.1/app/cdm/design/study_masters' \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}'
Response
{
"responseStatus": "SUCCESS",
"responseDetails": {
"limit": 1000,
"offset": 0,
"size": 3,
"total": 3
},
"study_masters": [
{
"study_master_name": "ABCP-2023-01",
"organization_name": "ABC Pharma",
"external_id": "",
"library": false,
"study_instances": [
{
"study_id": "OOZ00000009W001",
"study_name": "ABCP-2023-01_DEV1",
"study_label": "ABCP-2023-01_DEV1",
"study_external_id": "ABCP-2023-01",
"created_date": "2023-04-06T18:49:42Z",
"last_deployed_date": null,
"environment_type": "development__v",
"instance_status": "live__v",
"build_number": 15,
"highest_casebook_version": 1,
"sequence": 1,
"vault_id": 114107,
"vault_name": "My Development Vault"
},
{
"study_id": "-remote vault-",
"study_name": "-remote vault-",
"study_label": "ABCP-2023-01_TST1",
"study_external_id": "-remote vault-",
"created_date": "2023-04-17T18:49:42Z",
"last_deployed_date": "2023-04-20T13:49:42Z",
"environment_type": "test__v",
"instance_status": "live__v",
"build_number": 13,
"highest_casebook_version": 1,
"sequence": 1,
"vault_id": 114108,
"vault_name": "My TST Vault"
}
]
},
:
:
:
]
}
Notes
- This action can be performed from any Vault type (Development, Test, Prod, Training), but makes the most sense when from the Development / ‘Control Center’ Vault (where deployments happen from).
- Control center EDC Vaults drive the deployments Dev -> Test(s) -> Production
- Thusly, they have the best information on where a Study master stands, relative to its instances.
Endpoint
GET /api/{version}/app/cdm/coder/forms
Required Permissions
The following permissions are required to use the Retrieve Coding Definitions API:
- API Access
- View Study Design and/or View Library
The CDMS API Read Only and CDMS API Read Write roles grant these permissions.
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Query Parameters
Include the following parameters to filter the results:
| Name | Description |
|---|---|
study_master_name |
Optional A specific Study to filter the response to, or all is returned. |
organization_name |
Optional A specific organization in the Vault that holds the Studies. |
library |
Optional Boolean, true or false to filter to Library / Collection Studies only, or non-Library. When not provided, there is no filter on the property. |
This API supports pagination. By default, the page limit is set to 1000 records. The pagination parameters are:
| Name | Description |
|---|---|
limit |
The size of the result set on the page (as a positive integer). If omitted, the default is 1000. |
offset |
The starting record number for the result set (as a positive integer). If omitted, the default is 1. For example, if the limit is set to "100", and there are 150 possible records, use "101" as the offset to show results 101 through 150. |
Response Details
On SUCCESS, Vault returns a list of Study Masters (as JSON array entries). The information includes:
| Array | Name | Description |
|---|---|---|
study_master_name |
The overall name of the Study, often one to one with the name in the production instance. | |
organization_name |
The organization name the Study belongs to, is associated with. | |
external_id |
For the Study master, its external ID, often the same as the Study master name. | |
library |
true / false - whether the Study is classified for library purposes (or not) | |
study_instances |
study_id |
Study ID value, if local to the Vault being API'ed against. If the instance is in a remote Vault, this will not be known, so -remote Vault- is returned. |
study_instances |
study_name |
Study name value, if local to the Vault being API'ed against. If the instance is in a remote Vault, this will not be known, so -remote Vault- is returned. |
study_instances |
study_label |
The intended Study instance label in that target Vault. (as known in the development / control center Vault) |
study_instances |
study_external_id |
Study external ID value. If the instance is in a remote Vault, this will not be known, so -remote Vault- is returned. |
study_instances |
created_date |
Creation of the instance. This does NOT equate to when an instance was deployed 'to', just when setup for future deployment. |
study_instances |
last_deployed_date |
If deployed, when was the most recent |
study_instances |
environment_type |
The type of instance, e.g. development, test, training, production |
study_instances |
instance_status |
If live__v the instance has been deployed to, at least once. |
study_instances |
build_number |
The Study build number the Study instance contains. |
study_instances |
highest_casebook_version |
The Study design casebook version (highest) the Study instance resides. |
study_instances |
sequence |
The sequence of the instance |
study_instances |
vault_id |
The ID of the Vault the instance resides (could be the same Vault, or other) |
study_instances |
vault_name |
The name of the Vault the instance resides (could be the same Vault, or other) |
Create Study Master
Request - Create a new study master, with external ID provided
curl -X POST https://my-vault.veevavault.com/api/v24.1/app/cdm/subjects \
-H 'Accept: application/json' \
-H 'Content-Type: application/json' \
-H 'Authorization: {SESSION_ID}' \
-d '
{
"study_master_name": "ABCP-2023-01",
"organization_name": "ABC Pharma",
"external_id": "ABCP-2023-01",
}
'
Response - Create a new study master, with external ID provided
{
"responseStatus": "SUCCESS",
"study_master_name": "ABCP-2023-01",
"organization_name": "ABC Pharma",
"external_id": "ABCP-2023-01",
"library": false,
"study_instances": [
{
"study_id": "OOZ00000009W001",
"study_name": "ABCP-2023-01_DEV1",
"study_label": "ABCP-2023-01_DEV1",
"instance_created_date": "2023-04-06T18:49:42Z",
"last_deployed_date": null,
"environment_type": "development__v",
"instance_status": "live__v",
"build_number": 1,
"highest_casebook_version": 1,
"sequence": 1,
"vault_id": 114107,
"vault_name": "My Development Vault"
}
]
}
Notes
- The action is for creating a new Study master.
- A Study master generally encompasses all ‘instances’ of the Study (DEV1, TST1, TST2, prod instance, etc).
- However, the endpoint only creates a DEV1 Study (same Vault). TST and/or Production instances must be configured or created via the Tools -> EDC Tools UI.
- The action cannot be performed in any Vault other than the development / control center
Endpoint
POST https://my-vault.veevavault.com/api/v24.1/app/cdm/design/actions/create_study
Required Permissions
The following permissions are required to use the Create Study Master API:
- API Access
- API Read/Write access + Edit on Study Design and/or View Library
Headers
| Name | Description |
|---|---|
Accept |
application/json (default) |
Content-Type |
application/json |
Body Parameters
| Name | Description |
|---|---|
study_master_name |
Name of the new Study |
organization_name |
Name of the Organization the new Study should be classified under, in the Vault. |
external_id |
Optional If omitted, the Study master name value is used (usually the same value) |
library |
Optional Default is false, if not provided. Whether this new Study should be classified as a library Study (or not) |
Best Practices
Error Detection
Inner vs. Outer Response - Create Subject (1)
{
"responseStatus": "SUCCESS",
"subjects": [
{
"responseStatus": "FAILURE",
"errorMessage": "[Study Country] with name [Belgium] not found"
"study_country": "Belgium"
"site": "103",
"subject": "103-001"
}
]
}
Inner vs. Outer Response - Create Event Groups (2)
{
"responseStatus": "SUCCESS",
"eventgroups": [
{
"responseStatus": "SUCCESS",
"study_country": "United States",
"site": "101",
"subject": "101-001",
"eventgroup_name": "egIRT_INFO",
"eventgroup_sequence": 1
},
{
"responseStatus": "FAILURE",
"errorMessage": "[Subject] with name [101-021] not found"
"study_country": "United States",
"site": "101",
"subject": "101-021",
"eventgroup_name": "egIRT_INFO"
}
]
}
Strategy
If creating an integration into Clinical Data, what is your error detection overall strategy?
- Do you examine each response for success before moving on to a sequential / next call?
- What sort of alerting / process kicks off when there is an error?
- How do you avoid things falling through the cracks? (data out of synch with the EDC system)
- What about the mistake / update scenarios in the source system? What happens when your source data changes, mistake or otherwise, how do you push the data in, where an update might have a different course of action from the initial add?
Inner vs Outer Response Status
Watch the inner vs. outer success/fail statuses in API responses!
- The Vault API is designed such that most API endpoints allow multiple actions of that type in the same request.
- As such, the status of each entry in the request is embedded at the inner level,
responseStatus. - The outer
responseStatusis largely an indication that 'yes, you reached the server, and it attempted an action (or actions)'. - The examples to the right include:
- Attempt to Create Subjects (Casebooks) - one - that fails
- Attempt to Create Event Groups - two - where one succeeds, one fails
Study Design
Forms for Integration
For an inbound integration to Clinical Data:
- If possible, avoid Form designs that involve both user entered data and integrated data. Users will open the Form when the integration does not expect such, among many other timing related potential issues.
- If the data is semi-consistent from the upstream system from Clinical Data, consider:
- A template of Forms, 100% read only in their own dedicated Event Group.
- The approach can make use of Copy Event Group function in Studio to port the consistent Form designs to new Studies.
- Using cross Form derived rules, the read only remote system data can be pulled/copied or referenced in the main Study design.
- A template of Forms, 100% read only in their own dedicated Event Group.
Repeating Events
- A repeating visit in Clinical Data increments the
eventgroup_sequencevalues (1, 2, .. etc). - Although there is an Event sequence property on Event level, current system design yields that as a 1, always.
- Instead, the Event Group is used for both single repeating Events (e.g. Unscheduled), or series of Events (e.g. Day 1, Day 8, Day 15.. but by Cycle)
API Account for Integrations
Security Policy
- The suggested approach for the API account performing an integration is one where the Vault Security Policy has No Password Expiration checked.
- In this way, a recurring integration is not at risk of having an expired password upend the important push or pull of information.
Study Access and Training
- An API user is no different from any other Study user.
- Typically, users must go through / pass training before their Study access is truly enabled.
- For an API / system user, ensure to check the Ignore LMS Status, Assume Trained attribute on the account (typically). Otherwise the API user won’t find the Study (or Studies) intended.
- The action is not necessary if the specific Vault is not activated to required LMS training before Study access is granted. (e.g. test / sandbox Vaults)
Sequences
- The various design levels of a Study - Event Group, Form, Item Group - can repeat, or not repeat.
- When they do not repeat the Create / Upsert endpoints do not require the specific indication of, say:
"eventgroup_sequence": 1in the request body. - That said, consider always including these regardless, as it makes for a more consistent request/response match
Inspecting Before Doing
- Strive to inspect the existing Forms / Events before attempting an action to push data into the Study.
- Does the Subject even exist?
- Does the target area of data (Form, Event Group, etc) exist?
- If a Form is submitted, it must first be opened for edit via the Edit Submitted Forms endpoint before proceeding with the update of data using endpoints like Set Form Data or Upsert Form Data.
- TIP: The Combination Update Form Data endpoint is the most efficient approach (details below)
Create vs Upsert
- Various API endpoints started as only create actions (POST), where the intended location must *not exist for the action to be successful. (Exception: Set Form Data used for updating existing)
- Later releases included PUT action, which does no action when the location already exists (e.g. repeating Event Group, Form, or Item Group)
- The upsert option is a better option, since specific sequences can be indicated at the various design levels, with the result being either an add (if does not exist) or a skipped action.
- Update ‘2nd time in’ scenarios benefit from this approach.
Combination API for Form Data
- The EDC API's first release was #19R3*
- As releases progressed for the EDC API, endpoints were largely one to one with an action by an end user, and are used in a specific sequence. Examples:
- The sequence described above is common for an IxR vendor pushing data into EDC. But, it is a distinct API call to be made, each of the steps
- At API version 23R1, the Combination Update Form Data was introduced to allow for 'many in one' ability when updating Form data. Although it will not create Subjects, it will perform all of these in one request:
- Create Forms (when repeating and not present)
- Edit Submitted Forms (when the Form is currently in submitted state)
- Create Itemgroups (when repeating and are not present yet)
- Set Form Data
- Submit Form
Locked / Frozen Forms
- Frozen and Locked are not automatable through the API, by design. These were/are human decisions to stop actions from happening.
- Depending on the data location, Locked and Frozen statuses differ in ability to push data into Clinical Data:
| Area | Status | Add/Update Data | Add/Update Queries |
|---|---|---|---|
| Study | Locked | Disallowed | Disallowed |
| Site | Locked | Disallowed | Disallowed |
| Subject | Locked | Disallowed | Disallowed |
| Subject | Frozen | Disallowed | Allowed |
| Event | Locked | Disallowed | Disallowed |
| Event | Frozen | Disallowed (event date changes) | Allowed (on event date) |
| Form | Locked | Disallowed | Disallowed |
| Form | Frozen | Disallowed | Allowed |
Change Reasons
- For the update of a Form data, a
change_reasonis only used when there is an update/change to existing values, after there has been at least one submit of the Form. - Change reasons can be included for the initial set of data, but they are ignored, instead the default 'changes before submission' is used.
- That said, it is easier to send a
change_reasonwith all Form updates. In this way you don’t have to track necessarily that it's the 1st or later touch of the Form data. - Actions that require a
change_reasonwill default to a system reason, if not provided. Providing one is preferred to better identify the action in the remote system.
Jobs
- The Clinical Data job types that allow for starting, inspecting, and retrieving output follow the Vault platform job flow. That is, a job of Completed status might still potentially have issues (no output file available).
- Depending on the job type (Job Summary / Types), a more detailed log style information is usually included in the output.
- Both the Vault job log and the content of the job’s output should be inspected.
User Administration
- For updating of users with a EDC Vault, ensure the proper usage depending on the need:
- For deactivation of an account at the Vault level, this will remove the user from any Studies they otherwise would have had access to, in that Vault.
- For full deactivation of an account, this will remove the user from all* Vaults (thus all Studies in those Vaults) on that domain. Later reactivation of an account in such a state is then a two-step process. (update of domain status, then update of Vault memberships)
- For Study access deactivation, use the upload users endpoint (CSV or JSON), with a line indicating Study Status = Disabled, to remove access to a single Study.
- Specific site / country access does not need to be removed when disabling Study access, as later a re-activation in the Study might happen, returning the user to the state they were at deactivation in the Study.
- For (CSV or JSON):
- The structure of user information is the same as performing in the Clinical Data Users UI.
- The template can change from release to release as new properties are introduced.
- If some of the new properties are required, this could upend existing built integrations.
- The API version does NOT refer back and use older templates.
- Consult theClinical Data Help and experiment in pre release Vaults on coming releases when writing user administration integrations.
- The structure of user information is the same as performing in the Clinical Data Users UI.
- IMPORTANT - review the Types of Users sub-section above from the Retrieve Users.
